Rodent Gingival Tissue Culture in an Aging Experimental Model: A Pilot Study
Abstract
Background:
Gingiva acts as a barrier to prevent further invasion of pathogens in periodontitis. The gingival structure consists of epithelial tissue and connective tissue. As the aging process continues, there are several changes in the periodontium. Previous studies have tried to investigate the complex interaction between the host immune system and bacteria by using animal models, especially rodents.
Objective:
The aim of the study was to evaluate the effectiveness of collecting gingival tissue from the palate and retromolar pad.
Materials and Methods:
The aging experimental model had two age categories of male rodents of 18 and 58 weeks. Tissue was collected from the mandible retromolar pad and palate with full-thickness excision. Tissues were transferred to a complete medium at 4°C. Gingival tissue was cultured in a 37°C culture incubator at 5% CO2. Tissue proliferation was observed on the first, third, and fifth days using the hemocytometer. The cell metabolism rate between the two age categories was checked using the MTT Assay. Two-way ANOVA was used for statistical analysis.
Results:
Gingival tissues obtained from the experimental models of two age categories were alive, and proliferation was observed. The old rodent group showed no significant result in terms of cell morphology on the first vs. third day (p>0.05), but significant results were found on the first vs. fifth day and third day vs. the fifth day (p<0.05). The young rodent group showed the most significant morphology changes between days. In both young and old categories, no significant difference was observed in the cell metabolism.
Conclusion:
Rodent gingival tissue collection from the retromolar pad and palate was found suitable for tissue culture in the aging experimental study.
1. INTRODUCTION
Periodontitis is a multifactorial disease that can cause destruction of the overlaying apparatus, such as loss of junctional epithelial attachment, gingival recession, bone resorption, and ultimately, loss of the teeth. The complex interaction between the host immune system and bacteria leads to inflammation, and it plays a role in the periodontal apparatus destruction. Gingiva acts as a barrier to prevent the further invasion of pathogens, called the defense mechanism of the gingiva. The gingival structure consists of epithelial tissue and connective tissue [1].
Tribbel et al. suggested in vitro studies to investigate the interaction between host and bacteria using the culture animal models of gingival tissue. About 99% of rodents’ genome represents human cells [2]. Therefore, rodents are often used in experiments. It is a gold standard for animal studies experiments, as the ratio between human and rodents age is 1:9 [3, 4]. Periodontal studies observe gingival conditions either in vivo or in vitro. For in vitro studies, gingival tissue can be collected from the palate or the retromolar pad. The anatomy of rodents allows for easier tissue collection with adequate amounts of samples [5, 6]. Rodent’s oral anatomy in each quadrant consists of one incisor and three molars. Specific pathogen free is an ideal condition to grow and maintain the experiment animals, and it is built to protect the animals from viruses or pathogens so that they can grow healthy [7]. Former studies have evaluated the alveolar bone loss due to oral bacterial colonies in mice to model the periodontitis called the Baker mouse model. They have assessed the virulence factor of pathogens that caused periodontitis. The study used specific pathogen-free female BALB/c mice (10 weeks old) that were orally infected with A. actinomycetemcomitans and/or P. gingivalis strains. Assuming that P. gingivalis causes periodontitis in an animal model, the subgingival biofilm was modified to obtain enhanced virulence [8].
Aging is a normal process in which the body’s ability to respond to various stimuli and physical conditions is decreased. An altered immune response is a form of a functional decrease in the elderly so that they become more vulnerable when exposed to pathogens, as well as the tissue turnover. Tissue repair consists of 4 phases; hemostasis, inflammation, proliferation, and remodeling. Several studies state that there are differences in the level of tissue proliferation between young and old people, as well as their response to the inflammatory process [9-11].
Clinical manifestations of periodontal tissue in aging include decreased physiological tissue integrity, decreased number of elastic connective tissue fibers, dysregulation of collagen bonds, and decreased cellular quality [12, 13]. The function and structure of fibroblasts in the gingiva also change with the aging process. As individuals age, cells may show a gradual loss of replication potential and a lower response to growth factors, thereby reducing tissue repair capacity [7, 8, 14]. This is supported by the research by Pansani et al., who compared the number of fibroblasts in the young and the elderly, where the number of fibroblasts in the young was more than in the elderly [15].
Animal cell cultures were found as models for mimicking human gingival tissue. Therefore, this study aims to evaluate the effectiveness of collecting rodent gingival tissue from the palate and retromolar pad in an aging study design.
2. MATERIALS AND METHODS
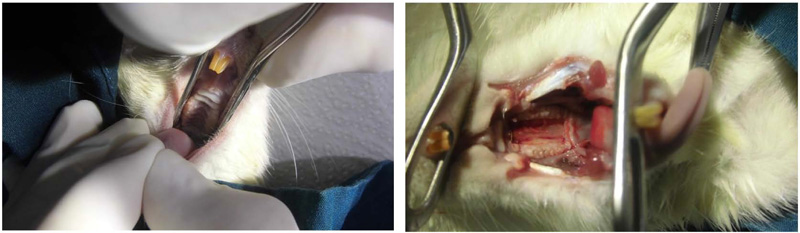
An aging experimental model was prepared by using two age categories of male rodents, 18 and 58 weeks old, with one rodent for each group. Euthanasia was performed by a veterinarian, followed by gingival tissue collection according to Bimana Indomedical Ethical Clearance (002-17-IR) using pentobarbital sodium. Gingival tissue was collected from the mandible retromolar pad and palatal with full-thickness excision sized 2mm x 5mm (Fig. 1). Tissues were transferred to complete Dulbecco’s modified Eagle’s medium (Gibco™ DMEM, High Glucose, HEPES, Massachusetts, USA), fetal bovine serum (BioSera, United States Origin), and antibiotic-antimycotic (Gibco, Massachusetts, USA), and stored at 4°C. Samples were then divided into two groups, group one for old rodents (58 weeks old rodents) and group two for young rodents (18 weeks old rodents). Tissues were cultured in a 75ml flask with a complete medium for about 20 days in a 37°C culture incubator with 5% CO2. The medium was changed every three days. Cells that reached confluence were then transferred to a 24-well culture plate, each well consisting of 104 cells/ml. Gingival tissue proliferation was observed on the first, third, and fifth days using the hemocytometer and microscope to evaluate the morphology of the gingival cells. The cell metabolism rate between the two age categories was checked using the MTT Assay; cells were placed in a 98-well culture plate and analyzed with an ELISA reader (Metertech, Taipei, Taiwan). Data analyses were performed using SPSS Statistics Version 26 (IBM Corporation, New York, USA), and two-way ANOVA was used for statistical analysis. Graphics were presented using GraphPad software 9.1.2 (GraphPad Software, San Diego, USA).
3. RESULTS
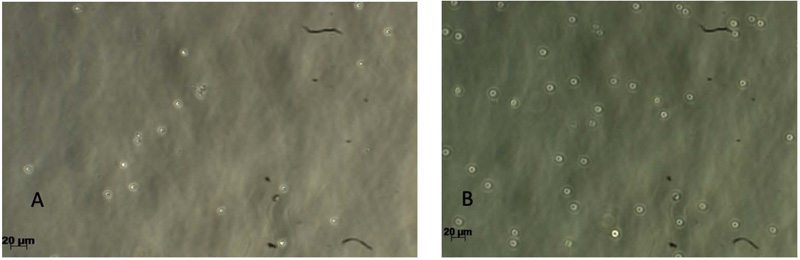
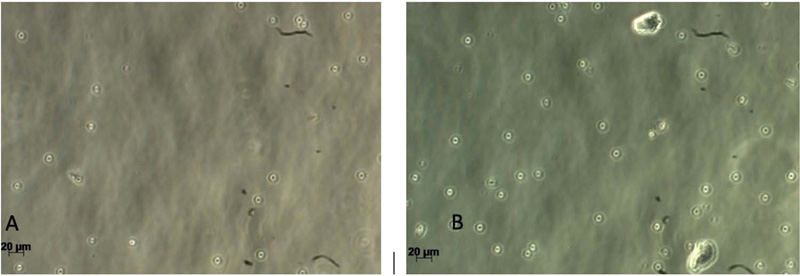
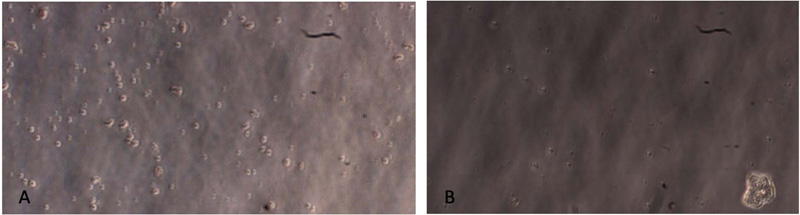
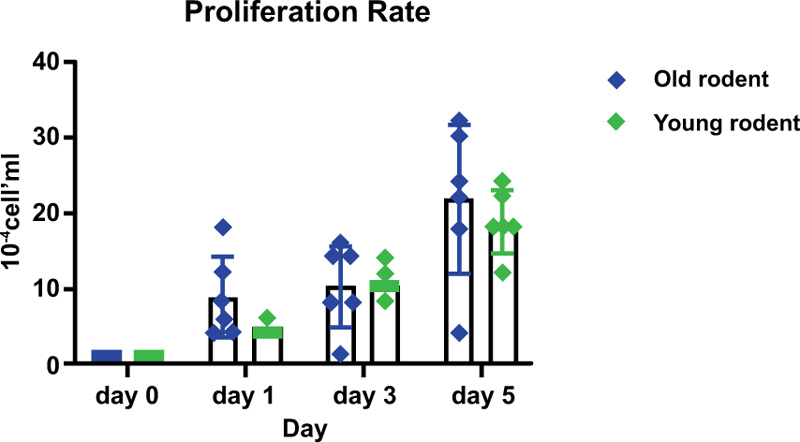
Gingival tissues from two age categories were alive, and proliferation was observed (Figs. 2-5). Microscopic images showed no significant changes in cell morphology on the first day, but there were morphological changes on the third and fifth days when cells started to attach and proliferate. Cell phology on the baseline, first, and third days looked similar; it had a defined outline and did not aggregate in groups (Figs. 2 – 4). On the fifth day, the old rodent group cells almost reached confluence compared to the young rodent group. Cells on the fifth day showed the most significant morphological changes, i.e., flat and thin shape, and overlapping in groups forming layers of mature cells (Fig. 5).
The old rodent group showed no significant results on the first vs. the third day, but significant results were found (p>0.05) for baseline vs. first, third and fifth days, as well as the first vs. fifth day and third day vs. the fifth day. The young rodent group showed a significant result (p>0.05) between days except for the baseline vs. the first day and first vs. the third day. The proliferation rate between old and young rodents showed no significant result on the first and third day, but significant results on the fifth day (Table 1 and Fig. 6).

| Cell Culture Duration | ||
|---|---|---|
| Rodents Age Categories | ||
| Day | Old | Young |
| 0 vs. 1 | 0.0178* | 0.4569 |
| 0 vs 3 | 0.0034* | 0.0019* |
| 0 vs. 5 | <0.0001* | <0.0001* |
| 1 vs. 3 | 0.9294 | 0.0882 |
| 1 vs. 5 | <0.0001* | <0.0001* |
| 3 vs. 5 | <0.0010* | 0.0125* |
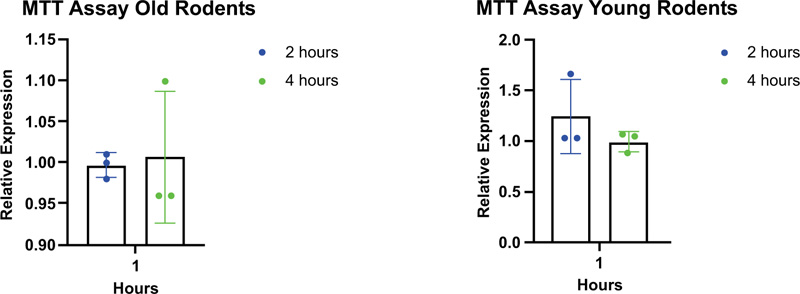
The MTT assay was used for the metabolism analysis, and the result showed no significant differences in cell metabolism between all age categories (Table 2 and Fig. 7).
| Rodents Age Categories | ||
|---|---|---|
| Duration | Young Rodent | Old Rodent |
| Two hours | 0.3114 | 0.3587 |
| Four hours | 0.3755 | 0.3892 |
4. DISCUSSION
This study indicates the proliferation rate of gingival tissue between young and old rodents to be increased significantly in prompt medium and certain duration time. This means that the proper environment and right procedure of tissue collection allow the cells to grow and run their physiological function. In this study, the aging model was represented by two age categories, young rodents (18 weeks old) and old rodents (58 weeks old). Cell cultures were established as models that mimic certain aspects of human periodontal tissue at the cellular level and pathogen-host interactions [16]. Rodents have unique characteristics that can be used to evaluate microbial and host responses to represent primate and human periodontal studies and are handy to acquire with different genes and microbial statuses. Rodents are also the most favorable mammalian model in aging research [6, 17].
Human aging studies have major limitations related to confined biological sampling and complications in intervention experiments. Therefore, for human aging and disease, animals have been used as models. This study used primary cells due to the limitation of cell availability with age differences. Dutzan et al. evaluated the gingival immune cell network by using mice gingival tissue from the palate and retromolar pad. The experiment represents the immune cell network well and provides good results for the research [18]. This study used the method suggested by Dutzan et al., wherein the gingival tissues were collected through a full-thickness incision and were cultured in a complete medium. This study also proves that cell culture using this method successfully allowed the growth of the gingival tissue in the old and young rodents, as indicated by the good cell proliferation rate. Although young rodents showed constant growth, old rodents showed exponential growth on days 3 and 5 only.
Testing cell viability is important to assure that the cells are adequate for the next step of the research. In this study, a cell viability test was performed using the MTT assay. Viable cells with active metabolism converted MTT into a purple-colored formazan product with absorbance. When cells died, they lost the ability to convert MTT into formazan; thus, color formation serves as a useful and convenient marker of the viable cells only. In this study, there have been observed no differences in metabolism in both the age categories. It means that young and old rodents have the same phase of cell metabolism. It is worth noting that MTT reduction is a marker reflecting viable cell metabolism [19].
The limitation of this study is the challenge of keeping the environment steady for the cells to grow. The study sample was smaller, and a larger sample could have made the experiment more reliable. For future studies, researchers can study the proliferation differences of the samples from different parts of the mucosa. This study used primary cells from rodents due to aging-related design; therefore, it took longer for the cells to proliferate compared to the cell line.
CONCLUSION
Rodent gingival tissue collection methods from the retromolar pad and palate are suitable for tissue culture in an aging experimental study.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Euthanasia was performed by a veterinarian, followed by gingival tissue collection according to Bimana Indomedical Ethical Clearance (002-17-IR).
HUMAN AND ANIMAL RIGHTS
No humans were used that are the basis of this study. The procedures followed complied with the standards outlined in the eighth edition of “Guide for the Care and Use of Laboratory Animals” (grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals_prepub.pdf; published by the National Academy of Sciences, The National Academies Press, Washington, D.C.).
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
This study was financially supported by grant PUTI 2020 provided by Research and Development Directorates Universitas Indonesia (NKB-1602/UN2.RST/HKP.05.00/ 2020).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


