Clinical and Histological Comparison of Extraction Socket Healing Following the Use of Autologous Platelet-Rich Fibrin Matrix (PRFM) to Ridge Preservation Procedures Employing Demineralized Freeze Dried Bone Allograft Material and Membrane
Abstract
Background:
The healing potential of platelet growth factors has generated interest in using Platelet-Rich Plasma (PRP) in ridge preservation procedures. A canine study was performed to determine if extraction sites treated with platelet-rich fibrin matrix (PRFM) exhibit enhanced healing compared to sites treated with non-viable materials.
Methods:
Four dog’s extraction sockets were treated individually with PRFM, PRFM and membrane, Demineralized Freeze-Dried Bone Allograft (DFDBA) and membrane, PRFM and DFDBA, and untreated control. Treatment sequencing permitted clinical and histologic evaluation of healing at 10 days, 2, 3, 6 and 12 weeks.
Results:
Healing was more rapid in the PRFM and PRFM and membrane sites. By 3 weeks those sockets had osseous fill. Sites containing DFDBA had little new bone at 6 weeks. By 12 weeks those sockets had osseous fill but DFDBA particles were still noted in coronal areas.
Conclusions:
PRFM alone may be the best graft for ridge preservation procedures. Advantages: faster healing, and elimination of disadvantages involved in using barrier membranes.
INTRODUCTION
Guided Bone Regeneration (GBR) procedures, employing non-vital bone grafting materials and membranes were developed to counteract the significant resorption of alveolar bone following tooth extraction [1-3]. Although ridge dimensions were improved with these procedures, the amount of augmention created at the time of GBR was not the amount of augmented bone present after several months of healing [4, 5]. The loss of augmentation is probably related, in part, to the lack of vascularity and viability of commercially prepared graft materials.
According to many investigators a better choice for regenerative procedures are autogenous bone grafts obtained from intraoral and extraoral sites [6, 7]. Extraoral grafts, such as the iliac crest marrow grafts, have excellent osteogenic potential but have the disadvantages of a second surgical site, with possible site morbidity, and reports of rapid resorption when used intra-orally [8]. Graft materials from intraoral sites also have potential site morbidity, significant resorption, and a limited graft volume that can be harvested [9]. Intraoral block grafts cells have a limited vitality, (24 hours), before the osteocytes undergo necrosis [10]. Autogenous particulate grafts from cancellous bone have relatively few cells and those obtained from cortical bone are almost devoid of cells.
Autogenous grafts that do contain vital tissue are those obtained from the centrifugation of a patient’s whole blood. In the late 1970’s, early 1980’s Hunt and Neighlton [11] described the separation of patient’s blood by centrifugation into three layers; 1) platelet-rich plasma (PRP), 2) platelet-poor plasma and 3) red and white blood cell layers. It is the PRP layer that is of interest for use in wound healing since this layer contains a concentrate of the patient’s platelets. These platelets have granules that contain growth factors that affect every cell and the formation of every tissue involved in the wound healing and regeneration of soft tissue and bone (Table 1). Marx et al. [12] first described the use of PRP in dental surgery. They claimed a radiographic maturation rate of bone grafts with PRP that was 1.62 to 2.16 times more rapid than grafts without PRP.
Growth Factors Found in Platelets in PRFM
| Factor | Target Cell/Tissue | Function |
|---|---|---|
|
PD-EGF (epidermal growth factor) |
|
|
|
PDGF, A+B (platelet-derived growth factor) |
|
|
|
TGF-β1 (transforming growth factor beta1) |
|
|
|
IGF-1,2 (insulin-like growth factor1,2) |
|
|
|
VEGF/ECGF (vascular endothelial GF) |
|
|
|
bFGF (basic fibroblast growth factor) |
|
|
In the preparation methods of PRP described by Marx et al. [12] and others, the coagulation process to obtain a gel was initiated with 10% calcium chloride and bovine thrombin. The introduction of an endogenous initiator of coagulation (usually bovine thrombin) in most of the commercially available methods of PRP preparation has the effect of causing rapid degranulation of platelets and almost immediate liberation of growth factors into the surgical area at the time of preparation [13]. Since growth factors have a limited time of effectiveness, immediate release of growth factors can only affect the immediate stages of wound healing and not the extended period of time needed for bone and soft tissue regeneration.
A platelet-rich fibrin material, which does not use bovine thrombin as an activator, has been described as a Platelet-Rich Fibrin Matrix (PRFM).* The proprietary process for PRFM preparation separates the blood cells from the platelets and plasma proteins, during an initial low speed centrifugation of a patient’s blood. A second centrifugation converts fibrinogen to fibrin in the presence of CaCl2 and the fibrin cross-links to form a matrix that contains viable platelets. Carroll et al. [14] have demonstrated, in vitro, that the viable platelets in PRFM released 6 growth factors in about the same concentration for the 7-day duration of their study.
Given prolonged growth factor presence it would be expected that PRFM treatment of an extraction socket might result in enhanced wound healing compared to those sites treated with non-viable graft materials. To test this hypothesis, a study was designed to compare PRFM*, to conventional GBR techniques utilizing demineralized freeze dried bone allograft (DFDBA) and bioresorbable collagen membrane, in a histologic study in dogs.
MATERIALS AND METHODS
The study was conducted on four mongrel dogs weighing 20 - 25kg. The experimental protocol was approved by the Institutional Animal Care and Use Committee of the University of Medicine and Dentistry of New Jersey. Four treatment modalities were evaluated and compared in each animal: 1) PRFM alone 2) PRFM with an osseous graft (DFDBA) incorporated 3) PRFM covered by a resorbable collagen membrane and 4) DFDBA covered by a resorbable collagen membrane.** In each dog, all of these modalities were individually employed in extraction sites immediately after tooth extraction. One site in each animal was included as an untreated control.
In order to assess extraction site healing over time, the times of extraction were staggered. The sequencing was designed so that each dog had the same seven teeth extracted in 2 surgeries. The timing of the surgeries in each dog captured the healing at 2 time specific intervals. The design also permitted each treatment modality and each time interval to be present in two different animals. Healing was evaluated at 10 days, 2 weeks, 3 weeks, 6 weeks and 12 weeks post-operatively.
PREPARATION OF THE PLATELET RICH FIBRIN MATERIAL (PRFM)
PRFM was prepared by collecting 9 ml of venous blood from each animal in a collection/separation tube (Tube 1) prior to beginning the extractions. This tube contained 1) a buffered tri-sodium citrate additive that prevents clotting during the separation stage and 2) a proprietary separation material. Tube 1 was centrifuged for 6 minutes at 1100g (RCF). Following centrifugation, PRP comprises the supernatant above the thixotropic separation gel and the red and white blood cell fractions are below the separation material.
Just prior to the graft material being placed, the PRP supernatant was transferred into a second tube (Tube 2) via a male/female Luer-Loc ‘Angel Wing’ transfer device. Tube 2 contains a calcium chloride additive that facilitates clotting of the PRP. Tube 2 was centrifuged for 15 minutes at 1450g (RCF). This spin caused the PRP to become a PRFM, which is a gel containing a 5x platelet/fibrin concentrate, as compared to the animal’s normal whole blood plus residual serum. The gel was easily divided so that [1] aliquot could be used for treatment with the gel alone and the 2nd aliquot mixed with DFDBA as the sequencing of the study design required.
Surgical Procedure
Preoperatively, a fentanyl patch was applied on a shaved portion of the dogs side and, glycopyrrolate (0.01 mg/kg), acepromazine (0.1 mg/kg), and morphine (0.5 mg/kg IV) were given as pre-medication. Thiopental (10-20mg/kg) given intravenously used for induction of anesthesia with isoflurance 1-3% inhaled via an endotracheal tube was used for general anesthesia. Local infiltration of lidocaine (2%) with epinephrine (1:50,000) was administered for hemostasis and to reduce postoperative pain.
Sulcular incisions were made around the teeth to be extracted. Full-thickness buccal and lingual flaps were reflected. The roots of the teeth were carefully and atraumatically extracted. Digital photographs were taken pre, during, and post surgery.
Treatment of each extraction site was performed according to the sequencing schedule previously described (Table 2). Where employed, the membranes were carefully positioned in accordance with the principles of human GBR procedures. Flaps were sutured with 4-0 Ethibond™ sutures.***
Schedule of Extractions
| 1st Surgery | 2 Week Healing | 3 Week Healing | 4 Week Healing | 6 Week Healing | 9 Week Healing | 12 Week Healing | ||
|---|---|---|---|---|---|---|---|---|
| Dog A | Extractions | 4 | 3 | End Point | ||||
| Dog B | Extractions | 3 | 4 | End Point | ||||
| Dog C | Extractions | 4 | 3 | End Point | ||||
| Dog D | Extractions | 3 | 4 | End Point |
Postoperatively the dogs were administered morphine (0.5 mg/kg) intramuscularly and a Fentanyl patch for analgesia and clindamycin (11 mg/kg PO, BID) for 5 days. The dog’s oral cavities were rinsed with a solution of 0.12% chlorhexidine rinse daily until sacrifice. The animals were weighed once weekly for the first two weeks post-operatively. A soft diet was provided for about 10 days post-surgery.
Histologic Preparation
Immediately after sacrifice the specimens were placed in 10% formalin solution. Following fixation, decalcification of the block sections was accomplished by immersion in undiluted RDO for approximately 5 - 8 days. Dehydration followed by paraffin embedding and microtome serial sectioning (5μ) was performed. Slides were stained with Hematoxylin and Eosin, or Mallory’s Trichrome.
RESULTS
Clinically, all of the 28 extraction sockets healed uneventfully. Soft tissue healing proceeded normally but appeared to be more rapid in the PRFM alone sites. By the end of the 12 weeks of the study all sites were clinically healed completely. Histologically, the healing was quite different and much more rapid for those sockets treated with PRFM alone or with a membrane as compared to those sites in which DFDBA was used as a graft. The most important observations are summarized below.
PRFM Alone and PRFM and Membrane (Fig. 1)
- 10 days - PRFM alone and PRFM and a membrane sockets were filled with an organized connective tissue and very little cellular infiltrate.
- 2 weeks - complete epithelialization of the wound had occurred. A well-organized fibrous connective tissue was seen with new bone growing from the entire periphery of the socket toward the center of the site.
- 3 weeks - the sites were completely filled with new bone.
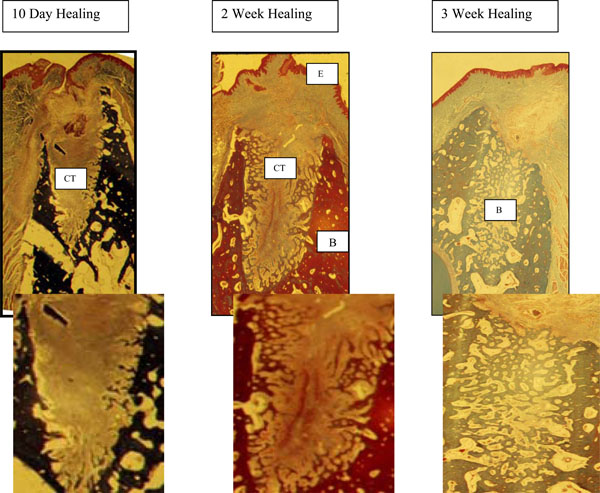
10 days - PRFM alone and PRFM and a membrane sockets were filled with an organized connective tissue (CT) and very little cellular infiltrate.
2 weeks - complete epithelialization (E) of the wound had occurred. A well-organized fibrous connective tissue (CT) was seen with new bone growing from the entire periphery of the socket toward the center of the site.
3 weeks - the sites were completely filled with new bone (B).
DFDBA and Membrane (Fig. 2)
- 3 weeks - the specimens have a thin epithelium overlying the extraction site with a slight discontinuity. The socket was still filled with non-vital DFDBA particles and dense inflammatory infiltration. No new bone formation was seen in the extraction socket.
- 6 weeks - epithelialization of the wound was not complete and non-vital osseous particles appeared to be present in the fibrous connective tissue overlying the area. The socket contained fibrous connective tissue but non-vital osseous particles surrounded by an inflammatory infiltrate could still be noted. There was a minimal amount of new bone observed in the apical third of the socket.
- 12 weeks - maxillary sites had complete fill of the socket with new bone and only a few bone chips were seen in the overlying connective tissue. In mandibular sites (above section – omit this reference to an above section), new bone filled most of the socket, but in the coronal third there was a cone shaped area of non-vital bone particles, surrounded by inflammatory infiltrate and connective tissue. This cone was narrow in the center of the socket becoming wider in the coronal portion of the area.
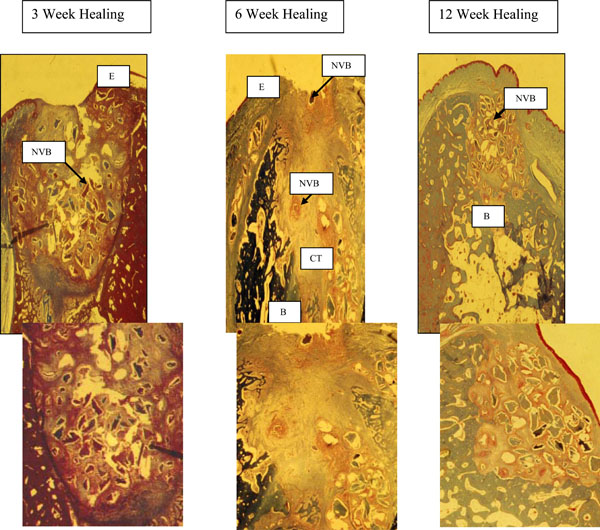
3 weeks - the specimens have a thin epithelium (E) overlying the extraction site with a slight discontinuity. The socket was still filled with non-vital DFDBA particles (NVB) and dense inflammatory infiltration. No new bone formation was seen in the extraction socket.
6 weeks - epithelialization (E) of the wound was not complete and non-vital osseous particles appeared to be present in the fibrous connective tissue overlying the area. The socket contained fibrous connective tissue (CT) but non-vital osseous particles surrounded by an inflammatory infiltrate could still be noted. There was a minimal amount of new bone (B) observed in the apical third of the socket.
12 weeks - maxillary sites had complete fill of the socket with new bone and only a few bone chips were seen in the overlying connective tissue. In mandibular sites (above section), new bone (B) filled most of the socket, but in the coronal third there was a cone shaped area of non-vital bone particles (NVB), surrounded by inflammatory infiltrate and connective tissue. This cone was narrow in the center of the socket becoming wider in the coronal portion of the area.
PREM with DFDBA (Fig. 3)

Sites treated with PRFM and DFDBA healed slower than those with PRFM alone or with a membrane but faster than those grafted with DFDBA and a membrane.
3 weeks – epithelization (E) is complete and some new bone (B) is noted in apical areas of the socket. Non-vital osseous particles (NVB) and an inflammatory infiltrate in the connective tissue (CT) is still the predominate feature.
6 weeks - new bone (B) is filling the apical half of the socket. The coronal portion of the socket still contains non-vital bone chips (NVB).
12 weeks - PRFM and DFDBA treated sockets exhibited the almost the same histologic picture at 12 weeks as the DFDBA and membrane areas except for fewer non-vital osseous particles, inflammatory infiltrate and connective tissue in the coronal portion of the socket.
Sites treated with PRFM and DFDBA healed slower than those with PRFM alone or with a membrane but faster than those grafted with DFDBA and a membrane.
- 3 weeks – epithelization is complete and some new bone is noted in apical areas of the socket. Non-vital osseous particles and an inflammatory infiltrate in the connective tissue is still the predominate feature.
- 6 weeks - new bone is filling the apical half of the socket. The coronal portion of the socket still contains non-vital bone chips.
- 12 weeks - PRFM and DFDBA treated sockets exhibited almost the same histologic picture at 12 weeks, as the DFDBA and membrane areas except for fewer non-vital osseous particles, inflammatory infiltrate and connective tissue in the coronal portion of the socket.
DISCUSSION
The advantageous effects of platelet-rich fibrin matrix (PRFM), upon healing of extraction sites in dogs, would appear to be due to the many proposed benefits of PRP growth factors which drive the wound healing processes and results in an improved rate and quality of bone formation, [15-17], enhanced vascular ingrowth [18,19], enhanced soft tissue healing [20, 21], mitogenic effects [22, 23] and increased bone regeneration [17, 24, 25].
Whether the proposed benefits of PRP result in superior healing has been investigated in humans and experimental animals. Although several reports have suggested that the addition of PRP to an osseous graft enhances healing in various applications [12, 26-28], other studies have indicated that adding PRP to a graft did not enhance the quantity or quality of new bone formation [29-33]. However, it is difficult to compare the animal studies since they used different animals, different study designs and platelet yields. The human studies were also varied in study design, numbers of patients, and application. Further, there were differences in PRP preparation, which would influence the content and concentration of growth factors in the PRP [34].
The PRP material used in this study is termed PRFM (Platelet-Rich Fibrin Matrix) and it differs from other commercially available PRP systems in that it does not use bovine thrombin or other exogeneos activators in the preparation process. The PRFM preparation process creates a gel-like matrix that contains high concentrations of non-activated, functional, intact platelets, contained within a fibrin matrix, that release, a relatively constant concentration of growth factors over a period of 7 days [14]. Unless there was a precipitous drop in concentration soon thereafter, there presumably would be sufficient concentration of these factors to influence the healing well into the normal sequence of introduction and functional maturation of the important healing elements.
The prolonged presence of growth factors in the healing sites may be the most significant factor that causes the much more rapid healing in the extraction sockets grafted with PRFM alone noted in this investigation. However, the prolonged presence of non-vital DFDBA particles and the accompanying inflammatory infiltrate noted during the healing through 12- weeks, was undoubtedly also a significant reason for delayed healing in the sockets where that material was used. Sites treated with PRFM alone had complete osseous fill of the extraction socket in 3 weeks, while sites treated with non-vital DFDBA still did not exhibit complete fill after 12 weeks. The fact that these DFDBA particles created an inflammatory/ foreign body reaction during healing of sockets has been reported in many studies. Becker et al. [35, 36] histologically evaluated human osseous graft cores and concluded that the over-riding histological characteristic of sites grafted with DFDBA or MFDBA was the retention of non-vital graft particles encapsulated with fibrous connective tissue. They suggested that the elimination of the non-vital bone particles from the wound was accomplished by macrophages or giant cells (foreign body reaction) or by sequestration, similar to the reactions observed in this investigation, not by resorption.. They concluded that non-vital osseous graft materials would delay healing and affect the quality of regenerated bone.
There may be other benefits when PRFM is used alone, without a membrane, as a graft. 1) Decreased time required to perform the socket preservation procedure. In the conventional GBR procedure using a membrane and DFDBA, properly placing and stabilizing the membrane after placement of the graft can take some time. When PRFM alone is used as the graft, the time required to place and stabilize the membrane is saved, since the membrane and its stabilization are not needed. 2) Elimination of complications and a reduction in ridge dimensions associated with membrane exposure. Membrane exposure of non-resorbable membranes has been shown to virtually eliminate any increase in ridge augmentation [37, 38]. Exposure of bioresorbable membranes may also result in a relative reduction in ridge augmentation, but the effects of exposure appear insignificant relative to those reported with non-resorbable membranes [5]. Obviously, if a membrane is not used or needed during the ridge preservation procedure, the potential disadvantages of membrane exposure are not a consideration. 3) “Better quality” of bone in healed extraction sites grafted with PRFM alone, because the sites will not contain non-vital bone chips. Non-vital DFDBA chips may take years to become resorbed by macrophages. Not only will the non-vital graft material delay normal bone formation, but the residual chips may weaken the host bone and/or create less than optimum bone next to dental implants [36]. 4) Possibly less bone resorption and therefore more ridge width and height after healing. Lekovic et al. [39] quantified the changes in alveolar width and height when a polygalactide/polylactide membrane was used for ridge preservation in intact extraction sockets (our Class I sockets). They recorded a 1.31 mm (17.79%) mean net loss of alveolar width and a 0.38 mm (11.59%) net loss of height after 4 to 6 months of healing. A more recent investigation by Iasella et al. [40] found that extraction sites treated with Demineralized Freeze Dried Bone Allograft (DFDBA) and a collagen membrane, incurred a mean net loss of 1.2 mm (13.04)% of pre-operative width. Other studies have shown an even greater net loss of width and height following GBR healing [4, 5]. The documented loss of alveolar height and width in these studies may be due to the inflammatory or foreign body reaction associated with non-vital bone particles. The inflammatory or foreign body reaction might cause resorption of the internal walls of the socket. In the coronal portions of the socket the width of osseous walls are thin, and internal resorption might lead to a reduction of the ridge after healing. With PRFM alone as the graft material, the healing process is more rapid and does not have an associated foreign body reaction adversely affecting the amount of bone formation.
CONCLUSIONS
PRFM may represent a simple and effective means of accelerating new bone growth in a variety of oral and dental applications without the disadvantages of barrier membranes, non-vital graft materials or exogenous thrombin associated with other PRP systems.
NOTES
* FIBRINET® Autologous System for Platelets and Fibrin, Cascade Medical Enterprises, Wayne, NJ.
** Bioguide Collagen Membrane, Osteohealth Co., Shirley, N.Y.
*** Ethibond Sutures, Ethicon Surgical, Sommerville, N.J.
ACKNOWLEDGEMENTS
This research was funded, in part, by an unrestricted grant from Cascade Medical Enterprises, Wayne, New Jersey.


