Antimicrobial Irrigation Solutions in Root Canal Treatment: A Glance at the Past, the Present, and the Future
Abstract
Background:
Many current concepts about irrigation solutions have evolved over time; a historical perspective of irrigation solutions and the reasons for their introduction to endodontic treatment is required. The authors of this work believe that a large number of unrecognised published works from the 20th century need to be brought to light so that researchers can acquire some important hints and insights into how those solutions were developed and used in the past.
In this paper, we investigate historical attempts to develop the optimal irrigation solution as well as the evolution of the scientific community's views on how to achieve this aim.
Methods:
A review of the literature related to irrigation solutions in endodontics was conducted using Scopus, Google Scholar, and Web of Science. Historical articles were identified through tracking citations of included articles and were obtained via the library of the University of Dundee.
Results:
Without the attempts of the past, we wouldn't be where we are today, including the role that several irrigation solutions played in endodontics before they were phased out. The observation that none of the currently available solutions had all of the properties that would make them ideal when used on their own led to the conception of the notion of mixing multiple types of irrigation systems, an idea that has since become widely popular.
Conclusion:
This study suggests pursuing two lines of inquiry: first, finding the best companion to sodium hypochlorite that produces no undesirable reaction precipitates; and second, maintaining the effort toward the development of a single irrigation solution that can effectively disinfect the canal without endangering the vital tissues. In general, and for some different possible combinations, there appears to be some light at the end of the tunnel, which is something that will hopefully be uncovered in the not-too-distant future.
1. INTRODUCTION
This work reviews the contribution of irrigation solutions to endodontic therapy throughout the years. Because many current concepts about irrigation solutions evolved in early times, a historical perspective of irrigation solutions and the reasons for their introduction to endodontic treatment will be provided. Throughout this analysis, it will become clear that irrigation solutions have or have had, a meaningful role in endodontics, regardless of their fate. Although few issues are fully resolved, there are convincing arguments that therapeutic benefits can be achieved with careful selection of irrigation solutions.
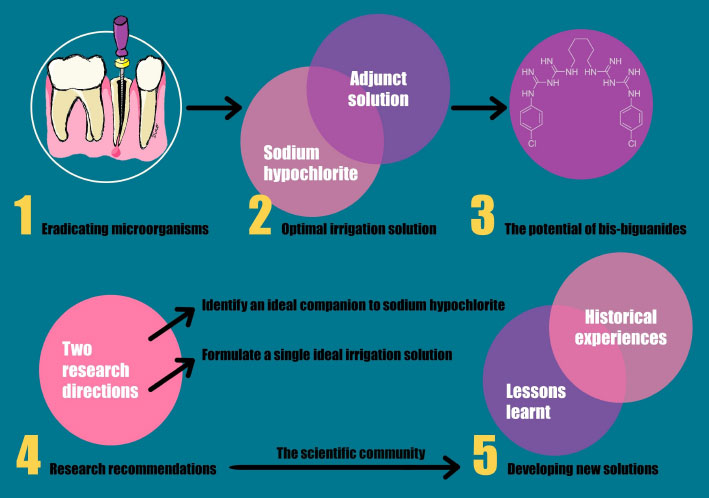
To better visualize the historical insights and key concepts in the development of endodontic irrigation solutions covered in this review, with an emphasis on the role of sodium hypochlorite and bis-biguanides, and the importance of continuing research in two primary directions to achieve optimal root canal disinfection, refer to (Fig. 1), which highlights the content of this review.
Inscriptions on excavated Ting dynasty ruins from 1400-1100 BC show the use of the Chinese character, which means “caries”. This character's design reflects the era's belief that caries were caused by the infestation of “worms” into teeth [1]. Similarly, during the French Revolution, extremely small “worms” with “a round head marked by a little black spot” were blamed for dental disease (2). Whilst others were not convinced of the presence of “worms”, it was a well-established view at that time that “acrid humour” was a major contributing factor to “fistulous abscesses”. To remove this irritant, treatment typically included trephination or drainage through the mucosa or root canal, as well as the application of various water-based preparations to the mucosa [2]. Poultices, cautery, and leaches were used, followed by gold foil root fillings that extended to the apex. However, the concept of sterility was not yet known, and it appears that root canal irrigation was not part of the practice of dentistry at the time.
The American Society of Dental Surgeons was founded in 1840 by leading dental practitioners, and the General Assembly of Maryland chartered the independent educational institution devoted exclusively to dentistry that same year. Other dental schools opened, but it wasn't until 1867 that the Harvard Corporation voted to establish the first dental school affiliated with a university and its medical school [3]. Concurrently, in Britain, the London Institute for Diseases of the Teeth opened in 1839, the first dental school was established in 1856, and the Royal College of Surgeons was granted a Royal Charter in 1859, allowing examinations in dental surgery [4]. Intracanal antiseptics, rubber dams, gutta percha, barbed broaches, and general anaesthesia were all introduced into dentistry.
Joseph Lister proposed in 1867 that “germs” exist in wounds [5]. Later research by Robert Koch in 1876 demonstrated that bacilli caused anthrax, which was quickly followed by the development of Koch's postulates, which would change the approach to medicine and dentistry [5]. Furthermore, Koch demonstrated in controlled laboratory studies in 1881 that pure cultures of bacteria could be eradicated by the use of hypochlorite-based solutions [6].
These developments had a direct impact on dental practice. It was widely assumed that sterilization could be accomplished by wiping the root canal with disinfectants and placing intracanal medicinal dressings. Thus, medicaments and broaches became the cornerstones of root canal therapy, and the appearance of periapical pathosis after opening into a pulp was attributed to the operator's lack of sterility [7]. All authors at the time emphasized the importance of using a rubber dam, which was invented in 1864 by Sanford Barnum of New York [5].
Taft was the first to advocate for frequent canal syringing [8]. This recommendation was based on the need to remove any irritants that may have been caused by pulp decomposition products. Irrigation was also advocated when arsenic trioxide, introduced by Shearjashub Spooner in 1836, was used to decompose the pulp [9]. Daily dressings with thorough canal irrigation (presumably with water) were encouraged at each visit, as was the use of “a deodorising agent, such as chloride of sodium” [8].
One author recommended pulp extirpation in a pool of eucalyptus, creosote, or clove oil, arguing that the oils would penetrate the canal as the nerve was removed [10]. Irrigation was also suggested for removing a firmly attached intracanal cotton dressing [11]. However, barbs and broaches were assumed to be the primary “cleansers” of the canal, and when combined with hot air from a syringe, complete canal debridement was thought to be possible [12].
The early literature describes various methods of cleaning the canal using various flushing agents and medications. One spectacular method involved injecting potassium and sodium metals into the canal, causing an “explosion” that effectively removed necrotic pulps [13]. This technique was still supported by at least one author in 1948, who claimed it was effective “in opening any multiple accessory apical foramenae present” [14].
Callahan (1895) proposed a 20%-50% aqueous solution of sulphuric acid applied on a cotton pellet and sealed in the tooth for 24-48 hours to widen root canals [15]. The canal orifice was then filled with a saturated solution of bicarbonate of soda, which caused an explosive effervescent reaction, purportedly neutralizing the acid and forcing debris to the surface. Another author suggested using agua regia (nitro-hydrochloric acid), which produced an effervescence similar to hydrogen peroxide [16]. This author rejected the use of sulphuric acid because “every instrument with which it came in contact underwent corrosion”.
Treatment of abscesses through openings of fistulas was routinely advised, with one method involving injection of a pure creosote and iodine solution into and through the fistula [17]. Following that, hydrogen peroxide-carrying barbs and broaches (Donaldson cleansers) were introduced into the canal [17]. The introduction of local anaesthesia, as well as the discovery of radiographs, first used for dental purposes by Otto Walkhoff in 1895, advanced the practice of endodontics by the late nineteenth century [5].
The theory of focal infection, introduced by Dr. William Hunter in 1910 and embraced by many dentists, had a significant impact on dentistry in the first half of the twentieth century [5]. This theory sparked a thirty-year period in which otherwise healthy teeth were extracted to allegedly cure systemic diseases. In fact, by the mid-1940s, this theory was still causing debate [18].
A textbook for dental students at the turn of the century described a technique for draining fistulae in which sodium peroxide was pumped (via a broach) through the canal until it reached the opening of the fistula [19]. Teeth with non-draining or “blind” abscesses were syringed with tepid water, dried, and then injected with hydrogen peroxide until all bubbling stopped. Sulphuric acid or trichloroacetic acid was then introduced into the canal via broaches and cotton and used to remove any remaining filaments.
Following pulp removal, canals were “washed out” with 3% hydrogen peroxide before being pumped with alcohol and allowed to dry. Because asepsis was now considered critical, it was recommended that a solution of sodium peroxide be pumped into the canals and left for a few minutes to “sterilize” the canal [19]. Hydrogen peroxide was said to have the added benefit of inducing haemostasis in the canal [20]. Furthermore, the carbon dioxide produced was thought to drive any remaining canal debris into the pulp chamber when accompanied by syringing with a saturated solution of sodium bicarbonate.
Infected “Septic” pulps could be cleaned with hot distilled water as well. Teeth that were too tender to manipulate were treated by syringing “Meditrina” and stirring with broaches [20]. Case (1905) also recommended routine and repeated syringing with 3% hydrogen peroxide for “septic” pulps. It's worth noting that Meditrina has been described as “sea-water charged with electricity,” with antiseptic and deodorant properties [19].
Barret (1901) proposed that the coronal access cavity be irrigated with hydrogen peroxide before pulp removal [21]. Following instrumentation of the canal with moistened broaches carrying dry sodium hydrate, also known as sodium hydroxide or “caustic soda” [22], and hydrogen peroxide, the canal was cleaned with a stream of water and a Donaldson cleanser. The author emphasized the importance of irrigant flowing through the apical foramen to eliminate any debris forced into the periapical tissues during preparation. Another author had previously noted that sodium hydrate (which is closely analogous to potassium hydrate) was highly soluble in water and extremely caustic [23].
With the need for a meticulous aseptic approach in mind, Black [24], a leading authority at the time, advocated irrigating “flooding” the root canal after canal preparation with medicaments such as oil cloves or other “sedative” oils. The use of “irritating germicides” to disinfect was, however, strongly discouraged [24]. Prior to filling, the canal could be irrigated with eucalyptol or oil of cajuput to remove any moisture from dentine by displacement, with this method of drying considered superior to hot air or hot instruments. Furthermore, because the oils dissolved gutta percha slightly, any remaining oil was thought to “stick” the material to the canal walls [24].
Colyer (1919) proposed several irrigation methods. Perforations could be treated by syringing hydrogen peroxide into the canal to stop the bleeding. When performing immediate root fillings, frequent irrigation with warm water removed debris [25]. Colyer also believed that using sodium peroxide and hydrogen peroxide alternately “sterilized” the canal, arguing that sodium peroxide in infected canals formed soap and thus eliminated the canal's “fatty” contents. The contents would then be ejected into the pulp chamber due to the rapid evolution of hydrogen peroxide. In cases where periodontitis was present, daily irrigation with antiseptics was recommended [25].
The importance of removing blood from the canal was recognized, and irrigation with alcohol or “Nature's greatest solvent, water” was advised [26]. It was suggested to use freshly distilled, boiled, or peppermint water (to which phenol had been added). If desired, sodium chloride could be added. For an abscess with a sinus, the same solutions or “any bland solution” were recommended [26, 27], as well as vigorous irrigation via the canal until forced through the sinus. However, the “common practice” of using a hydrogen peroxide solution was condemned [26]. A non-canal approach involving tissue incision and thorough irrigation of the wound with the salt solution was proposed [28]. The canal would then be dressed with a medication.
One astute dentist, Wilkinson, experimented with various “irrigation solutions”, including papain, an enzyme found in the leaves, fruit, milk, and seeds of the Pawpaw [29]. However, these solutions were only recommended as a replacement for unavoidable inadequate instrumentation. Grossman and Meiman (1941) conducted the first experiments on irrigant solutions, investigating the solvent action of several agents such as enzymol (which contains gastric juice and proteolytic enzymes), galactonic lactone (a mucin solvent), double-strength chlorinated soda solution, potassium hydroxide (20%), sodium hydroxide, and others [30]. The most effective pulp tissue solvent in this study was found to be double-strength chlorinated soda solution. Dakin (1915), however, discussed the solvent property of sodium hypochlorite in an article on the treatment of infected wounds much earlier [31].
Grossman's (1943) recipe for double-strength chlorinated soda solution called for 18 gm of monohydrated sodium carbonate, 26 gm chlorinated lime, and 4 ounces of water [32]. However, there appears to be some debate in the literature about how the term “double strength” should be understood. While Svec and Harrison (1977) define the term as requiring more than 5% available chlorine [33], Gordon, Damato, and Christner (1981) define the term as requiring 3% available chlorine [34]. Grossman’s paper (1941) resulted in a seminal article recommending the use of double-strength sodium hypochlorite combined with hydrogen peroxide to wash out pulp tissue fragments and dentinal shavings after mechanical instrumentation [32]. The effervescence produced by this combination was claimed to “follow the line of least resistance, namely, toward the mouth of the canal and into the pulp chamber”. As a result, dentists were advised to always irrigate with sodium hypochlorite at the end to avoid pressure buildup caused by any remaining hydrogen peroxide. Grossman (1943) also recognized that inorganic acids had drawbacks such as periapical tissue irritation [35].
Endodontics experienced a revival around this time, with the establishment of the American Endodontic Society in February 1944, which established endodontics as a distinct field within dentistry. In 1946, the Society began publishing its own journal [36], providing a vehicle for the development and dissemination of new ideas, as well as a more standardised treatment approach. This work will now discuss the research and ideas on endodontic irrigation solutions that have emerged since these times.
To adequately disinfect the root canals, the irrigant must be carefully selected because it complements the mechanical instrumentation used to remove microorganisms in the so-called chemo-mechanical preparation. The ideal irrigant is nontoxic, non-antigenic, non-carcinogenic, capable of penetrating and disinfecting dentinal tubules, and has a long-lasting antibacterial effect. It should also remove the smear layer and dissolve pulpal tissue remnants [37]. There is no ideal irrigation solution at the time of writing this manuscript. Some microorganisms will inevitably be left behind due to their ability to become incorporated into the smear layer and the micro-gaps and irregularities that are commonly found within the root canal system space. For many years, the two most commonly used root canal irrigant chemicals have been sodium hypochlorite and chlorhexidine at various concentrations. They do, however, have some drawbacks, so researchers are looking for an ideal irrigant with superior compatibility and antimicrobial activity.
A variety of endodontic irrigation systems have been proposed throughout the last few decades. Many of these agents, which are prescribed based on anecdotes and personal clinical experience, have not been submitted to rigorous investigation, and when they are, they fail in certain aspects, rendering them obsolete. Other agents have only been investigated by a few researchers and require more independent evaluation before routine usage, at least as an adjunctive, may be advocated. While many of the irrigation solutions to be discussed might possess distinct beneficial properties, none have proved capable of fulfilling the requirements of the “ideal irrigant’ [37]. In reviewing the literature, it would be helpful to shed light on some of the outmoded irrigation systems that were once thought to be effective and safe. It will become apparent in this loosely chronological review that none of these irrigation solutions provide significant advantages over sodium hypochlorite. More emphasis will be placed on the action against E. faecalis in the new era of materials that have appeared in the last two decades. This will not apply to older materials because E. faecalis was first discovered in root canals in 1964 and was not given much attention at the time [38]; it wasn't until 1998 that this bacterium species was recognised for its role in endodontic treatment failures [39].
The objective of this historical review study is to investigate and provide a comprehensive understanding of the development and evolution of endodontic irrigation solutions throughout the 20th century and earlier. By examining the scientific community's historical attempts to develop optimal irrigation solutions and exploring the reasons for the introduction and usage of specific solutions in endodontics, the study aims to shed light on the progress made and lessons learned from past experiences. This in-depth analysis will enable researchers and practitioners to gain valuable insights into the development and usage of irrigation solutions in the past, ultimately informing future research directions and the continuous improvement of endodontic treatment techniques.
2. METHODOLOGY
In this historical review article, the methodology focused on investigating the development and evolution of endodontic irrigation solutions. To achieve this, a comprehensive literature search was conducted using Scopus, Google Scholar, and Web of Science databases. The search terms and synonyms included irrigation solutions, endodontics, history, and development, which were combined in specific search strings to refine the results. The inclusion criteria consisted of articles discussing the historical development and usage of endodontic irrigation solutions, published in the 20th century or earlier, and available in English, French, or German. Exclusion criteria aimed to eliminate articles not focused on the topic, lacking historical perspective, providing no original data or insights, or not being available in the specified languages. To ensure the inclusion of relevant historical articles, citations within the initially identified articles were tracked, uncovering additional resources not indexed in the searched databases. These historical articles, including those in French and German, were obtained via the library of the University of Dundee, thus providing a comprehensive understanding of the evolution of endodontic irrigation solutions.
3. IRRIGATION SOLUTIONS IN ROOT CANAL TREATMENT: A HISTORICAL OVERVIEW AND PROSPECTS
3.1. Hot Disinfectants – Mercury Phenylborate (1940s)
Irrigation with hot (60°C-70°C) disinfectant solutions of mercury phenylborate, invert soap, organic mercury, or monomolecular mercury was recommended by Prader (1948) [40]. The author claimed that the higher the temperature of the solution, the shorter would be the time required to obtain sterility. Although that was the only report of using mercury phenylborate in dental applications, mercury use in dentistry has been gradually phased down over the years. Furthermore, evidence in the middle of the 1990s started to strongly contradict the unsubstantiated opinions pronounced by various dental associations and related trade organisations regarding the safety of mercury to dental personnel and their patients [41].
3.2. Enzymes – Tryptar and Varidase (1950s)
The rationale behind the use of enzymes in solution for irrigation was to enhance the natural defensive response of the host. Examples were Tryptar and Varidase. Based on eight cases, Auslander and Roth (1953) recommended Tryptar as an irrigant and medicament, and a safe and effective necrotic tissue solvent. The authors described Tryptar as a “highly purified crystalline trypsin derived from mammalian pancreas” [42]. Indication for use included “selective physiologic debridement of necrotic tissue”. Although one patient experienced an allergic reaction following application, the authors felt that this could be obviated by prior administration of an antihistamine agent.
Varidase, a combination of streptokinase and streptodornase, both extracellular enzymes elaborated by S-haemolytic streptococci, was endorsed as an irrigation solution and medicament by Blechman (1953) [43]. He asserted that streptokinase lysed clotted blood, while streptodornase catalysed the breakdown of deoxyribonucleoprotein, which was speculated to constitute 30%-70% of the sediment of thick purulent exudates. Golden and Musgrave (1954) claimed good clinical results with Varidase, which they asserted would enhance and complement the natural defence mechanisms of the surrounding tissues. No allergic reactions occurred during 13 cases [44].
Recently, the antibacterial effectiveness of an irrigation regimen including 1% trypsin and 2% chlorhexidine against E. faecalis species was demonstrated [45]. The presently available data and material safety data for trypsin indicate a modest irritating effect on respiratory mucosal membranes. Trypsin has a favourable influence on wound healing as well [46].
Trypsin is a well-known proteolytic enzyme that degrades proteins into peptides [47]. Trypsin, when used as an irrigant, has been shown to have antimicrobial and biofilm-disrupting potential, though not to the same degree as sodium hypochlorite [48]. Results from a study comparing the effectiveness of chlorhexidine, sodium hypochlorite, and trypsin against E. faecalis in the root canal system showed that trypsin followed by either chlorhexidine or lower concentrations of sodium hypochlorite produced satisfactory antibacterial outcomes. However, more research is needed to determine how trypsin affects the physical properties of root dentine, its compatibility with other irrigation solutions, and its potential in the disinfection of root canal spaces [48, 49].
3.3. Urea (1960s) and Urea Peroxide (1980s)
A 30% urea solution was recommended as an “excellent root canal wash, following vital pulp removal with haemorrhage” [50]. It was considered to be non-toxic and non-irritating to the periapical tissues. Further, it was claimed that 30% of urea removed clotted blood from the canal walls, in addition to deodorising putrescent suppurating canals [43]. Penick and Osetek (1970) described a 30% saturated solution of urea as a non-toxic, mild solvent of necrotic tissue and pus, with mild antiseptic properties, which was not as effective as 5% sodium hypochlorite [51].
The crystalline powder urea peroxide is composed of urea and hydrogen peroxide. It is unstable in aqueous form but becomes stable when combined with an anhydrous glycerol vehicle, which then allows dissociation into urea and hydrogen peroxide at a slower rate [51]. To find an antiseptic compound more stable than 3% hydrogen peroxide, Stewart, Cobe, and Rappaport (1961) used the presence of growth-free cultures to assess the germicidal properties of Gly-Oxide compared to aqueous hydrogen peroxide [52]. The authors believed that Gly-Oxide, a solution of 10% urea peroxide in a vehicle of anhydrous glycerol, was a more stable antiseptic compound than hydrogen peroxide, and also had lubricant properties.
A previous study by one of the authors [53] attempted to assess the action of 3% Gly-Oxide compared to 3% hydrogen peroxide, by measuring the release time and height of foam formed when these agents were exposed to citrated human blood. Cobe reported that the evolution of bubbles was immediate with 3% hydrogen peroxide, whereas Gly-Oxide produced more “compact” foam which developed gradually and lasted longer. Cobe suggested that the longer the period of oxygen release, the greater the chance for bactericidal action to occur.
Stewart, Cobe, and Rappaport (1961) suggested the combined use of Gly-Oxide and 5% Sodium hypochlorite to increase effervescence and bactericidal effect [52]. However, Cohen, Stewart, and Laster (1970) found this combination increased dentine permeability to methylene blue dye [54]. Brown and Doran (1975) found that the combination of Gly-Oxide and 5% Sodium hypochlorite was able to reduce the height of dentine particles in a simulated canal when the needle tip was positioned 2 mm from the dentine debris [55].
Weine (1982) claimed that because of its low toxicity, Gly-Oxide was an “excellent irrigant” for teeth with wide apices, where a risk existed of escape of solution into the periapical area [56]. Its lubricant properties were also considered effective in narrow or curved canals. An in vitro study by Foley et al. (1983) tested the effectiveness of Gly-Oxide in the elimination of Bacteroides melaninogenicus from the root canal system [57]. Gly-Oxide was effective, but not when diluted. Using SEM, Rome, Doran and Walker (1985) observed that Gly-Oxide in conjunction with Sodium hypochlorite did not remove the smear layer [58]. Further, there was no difference in smear layer formation using Sodium hypochlorite alone or in combination with Gly-Oxide.
In current times, urea has become a prominent component of numerous endodontic lubricants, including RC-Prep, Glyde File, and FileCare EDTA, which all contain 10% urea peroxide as one of the main constituents. Although these formulations are used as instrument lubricants, they are also hypothesised to impede the oxidation of EDTA by the action of urea peroxide and to have an antimicrobial activity due to the oxidising antibacterial effect of urea peroxide [59].
3.4. Local Anaesthetic (1980s)
Although there appears to be no published research on the efficacy of local anaesthetic as an irrigant, there has been a reference to it as a bland, sterile flushing agent [60, 61]. Topical anaesthetic has been suggested as a lubricant to aid mechanical preparation [62].
Mayne (1959) recommended sterile isotonic solution, such as local anaesthetic warmed in hot water, as an irrigant during the removal of vital pulps [63]. It was speculated that a non-isotonic solution could cause the rupture of red blood cells, thereby allowing the breakdown of products of haemoglobin to enter the dentinal tubules, and result in subsequent tooth discolouration. Local anaesthetic should not be used as an irrigant with the apex locators as it might interfere with measurement readings [64].
3.5. Chloramine-t and Azochloramid (1980s)
The organic chloramines are produced by the reaction of HOCl with an amine, amide or imide [65, 66]. They are not stable in water and release chlorine slowly. Chloramines have been in existence for many years as a general skin and wound disinfectants [67, 68]. Because chloramines contain a variable N-chloro substitute, there are several chloramines available, one of which is chloramine-T. However, the dental literature frequently refers to “chloramine-T” and “chloramine” interchangeably.
Chloramine-T (sodium p-toluene sulfonchloramide) is a white crystalline powder that contains approximately 45% available chlorine [66]. It is formed by the combination of sodium 4-toluene sulfonamide and HOC1 [69]. Although, like hypochlorites, chloramine-T hydrolyses to form HOCl, Penick, and Osetek (1970) considered it to have a considerably slower bactericidal action than the hypochlorites [51]. Nicholls (1962) found no difference in bactericidal effectiveness between chloramine-T and hydrogen peroxide/Sodium hypochlorite [70].
Luebke (1967) claimed that 4% chloramine-T was a more stable and less irritating, but less effective, solution than Sodium hypochlorite [71]. However, another writer believed that chloramine-T was more germicidal, more stable, and less irritating than Sodium hypochlorite but offered no supporting evidence [72]. Attala and Calvert (1969) provided a formula for the preparation of chloramine-T irrigant by dissolving 134.4 gm chloramine, 26 gm sodium chloride 26 gm in 3.3L water [73]. These authors found that chloramine-T produced a mild inflammatory response when applied to dog’s eyes and injected into guinea pig subcutaneous tissue. In contrast, Wennberg (1980) found 5% chloramine-T produced severe tissue reactions in the thigh muscle of rabbits [74].
Martin (1979) claimed that 3%-5% chloramine-T possessed antiseptic activity and lower toxicity than Sodium hypochlorite, but a poorer solvent activity [60]. Spangberg (1985) believed chloramine-T possessed “excellent antimicrobial qualities”, and a long shelf life if stored in a cold place and away from light [75]. Ingle et al. (1985) commented that its solvent ability was poor [76]. Reichardt (1973) advocated the use of 1%-2% chloramine-T heated to 60°C when irrigating lesions of periodontal and endodontic origins [77]. The combination of 10% chloramine-T with EDTA produced precipitation of toluene-sulfamide, which were colourless crystals and “probably innocuous” [78].
Chloroazodin, or Azochloramid, is an organic chloramine that is slightly soluble in water [66]. It is light sensitive and can exert a mild but sustained antimicrobial activity, however, it is less reactive with organic matter than other chlorine compounds such as chloramine-T and Sodium hypochlorite. Contact with metals accelerates its decomposition. Ursini (1947) described Azochloramid as a compound that slowly liberated chlorine and suggested it as a dressing or irrigation solution [79]. Azochloramid was later shown to exhibit a very high tissue-irritating potential [80].
Even though the scientific community now acknowledges that Chloramine-T retains accessible chlorine for much longer periods than sodium hypochlorite when tested on organic substrates [81], chloramine-T has never gained widespread acceptance in root canal treatment and both chloramine-T and Azochloramid appear to be less effective as antimicrobial agents than hypochlorite at comparable concentrations [37].
3.6. Aminoacridine (1950s)
Acridine is the nitrogen compound benzquinolone which is structurally related to the antimalarial quinolines [82]. Quaternarization of acridine increases bacteriostatic activity, as the most highly ionized cationic compounds are the most active. At 37° C and pH 7.3, 9-aminoacridine is in 99% cationic form. The early literature [83, 84] refers to 5-aminoacridine, which was commonly used as a mucous membrane antiseptic [85]. A 1:1000 solution of Monacrin (5-amine acridine hydrochloride), described as being active in the presence of serum proteins and pus, was recommended as an irrigant by Lyell (1951). As a result of the inability of this agent to act effectively on Candida albicans and Pseudomonas pyocyanea, Jolly and Sullivan (1956) added other agents to form the following mixture: Monacrin (5-aminoacridine hydrochloride) 1:500, Cetavion (alkyl trimethyl-ammonium bromide) 1:500, Methyl NIPA ester (methyl parahydroxybenzoate) 1:500, and Propyl NIPA ester (propyl parahydroxybenzoate) 1:3000, titrated to pH 6.0 with N/10 NaOH. This combination formed a solution that was used routinely in the 1950s. To increase the bactericidal effect of the mixture, the addition of 1:500 chlorhexidine digluconate was suggested by Atkinson and Hampson (1964) [86]. Jurecko (1974) examined the effectiveness of 9-aminoacridine alone, and in combination with benzalkonium chloride (a quaternary ammonium compound) [85]. It was found that 9-aminoacridine had antibacterial activity against Staphylococcus aureus, Staphylococcus epidermis, E. faecalis, and Candida albicans, but not against Streptococcus mitis. Application of the solution to rabbit eyes showed a mild response to 9-aminoacridine when compared with eugenol and parachlorphenol. Jurecko believed that the antibacterial action and apparent low toxicity of 9-aminoacridine could provide the appropriate properties required for an irrigation solution, and its routine use for a final wash before obturation was also advocated [85]. The addition of benzalkonium chloride was thought to provide greater antibacterial action against staphylococci and Candida albicans and, potentially, slightly increased eye tissue irritation.
Pressure irrigation of periapical tissues using 9-aminoacridine via the root canal was suggested by Breese (1950) [87]. Schmitz (1980) recommended the routine use of 9-aminoacridine as an endodontic irrigant. He believed it resisted inactivation by pus, secretions, and body fluids and had a low order of toxicity [88]. However, its mechanism of antimicrobial action, which purportedly involved inhibition of bacterial cell protein and DNA synthesis by frame-shift mutation, was extrapolated to be non-carcinogenic from studies performed on a “similar acridine derivative proflavine”. This might not be a valid assumption. 9-amino acridine is inactivated by soap, and stains carious dentine brown while intensifying the yellow colour of surrounding dentine. This could create aesthetic problems following the irrigation of anterior teeth [88].
Aminoacridine was utilised as an endodontic intracanal medicament at the University of California School of Dentistry beginning in 1950 and continued for another 25 years until 1975 [85]. The reason for its discontinuation is Seeman's 1975 report, which advised against its use due to its mutagenic potential [89].
3.7. Quaternary Ammonium Compounds
Quaternary ammonium compounds are cationic surface-active agents characterised by structural balance between one or more hydrophilic and hydrophobic centres [65]. The mechanism of quaternary ammonium compounds’ action includes causing the lipid bilayer membranes that make up the bacterial cytoplasmic membrane and the outer membrane of Gram-negative bacteria to malfunction so that they are unable to perform their normal functions. At first, the negatively charged bacterial cell walls interact with the positively charged ammonium group, which then disrupts the electrical equilibrium of the cell by displacing the divalent cations. In addition, the hydrophobic substituent attaches to the hydrophobic membrane core, which has the effect of making the membrane less fluid. In the end, the membrane is deprived of a great deal of its osmoregulatory and physiological functions. The outcome of this condition includes ions, protons, and other components of the cytoplasmic membrane escaping the cell, which ultimately leads to the death of the cell [90].
3.8. Quaternary Ammonium Compounds: Biosept and Zephiran
Strindberg (1957) first advocated the use of a “non-toxic bactericidal quaternary ammonium compound” 0.1% Biosept (cetyl pyrimidinium chloride) in preference to sulphuric acid, which was described as both toxic and corrosive [91]. It was suggested that no significant differences existed in the “cleansing effect” or “risk of exacerbation” between the two solutions. Grahnén (1963) compared 0.1% Biosept with a polyantibiotic, Nebacetin (containing neomycin and bacitracin), in water. No obvious difference in efficacy was found from bacteriologic culturing and assessment of clinical results (tenderness, pain, swelling, and presence of exudate) [92]. A later study by Engstrém and Spangberg (1969) found 1% of Biosept had a “marked antimicrobial effect” in vitro against Staphylococcus aureus, E. faecalis and candida, but not against Pseudomonas aeruginosa. However, the authors claimed that the potentially toxic effect of 1% Biosept against HeLa cells could not justify its use as an irrigant [93].
Using cultures to assess effectiveness, Shapiro, Heling and Erb (1966) examined another quaternary ammonium compound, Zephiran as an irrigant (0.133% solution alkyl dimethyl benzylammonium chloride by Winthrop Laboratories, USA) [94]. Due to reports of Zephiran being mildly irritating to oral tissues, it was advised that the material be used in conjunction with paramonochlorophenol as an intracanal medication [80]. Zephiran is now a routinely used irrigating agent for eliminating Staphylococcus aureus in infected orthopaedic wounds [95].
3.9. Quaternary Ammonium Compounds: Salvizol and Solvidont
Since 1977, reports on other quaternary ammonium compounds, Salvizol and Solvidont (DeTrey, Dentsply, Zurich, Switzerland) have appeared in the dental literature. Kaufman et al. (1977) described Salvizol, bis-dequalinium acetate, as a “new chelating solution” which possessed both inorganic solvent properties (due to its acetate group) and possible organic solvent properties, thereby providing a superior irrigating solution to EDTA [96]. In a later article by Kaufman et al. (1978), assertions were made that Salvizol had definite organic and inorganic solvent properties, as well as a low toxicity, wide-ranging bactericidal qualities, lubricant and surfactant features plus biological compatibility, and a neutral pH [97]. Zach and Kaufman (1983) reported that Salvizol outperformed EDTA in cleansing the apical one-third of the canal in an SEM investigation. Salvizol, on the other hand, did not degrade root canal dentine collagen [98]. Other SEM studies confirmed that Salvizol failed to remove the smear layer or effectively remove debris [99, 100].
Koskinen, Meurman, and Stenvali (1980) found Salvizol did not interfere with the surface morphology of uninstrumented root canal dentine of young premolars [101]. Solvidont was developed as an “endodontic kit” based on Salvizol, and consisted of bis-dequalinium acetate in three different concentrations and viscosities: 0.5% bis-dequalinium acetate working solution; 0.05% bis-dequalinium acetate irrigation solution; and 0.5% bis-dequalinium acetate intracanal medication paste. Kaufman (198l) reported that bis-dequalinium was efficient in promoting the healing of periapical lesions within a short time after treatment [102]. However, it appears that there was some confusion over this product. Griffiths and Stock (1986) found “the cleaning efficacy of Solvidont was unsatisfactory”, and found sodium hypochlorite to leave less debris in instrumented root canals than Solvidont [103]. In two in vitro studies it was reported that Salvizol “has biocompatibility and cleansing properties that make it potentially useful in clinical endodontics” and that Solvidont was less irritating than 1% Sodium hypochlorite [104, 105]. In the 1980s, 0.6% Salvizol was considered optimal for use as a root canal irrigant. The antimicrobial and toxic effects of the same solutions were examined in vitro [105]. Cytotoxicity tests were performed using labelled L929 mouse fibroblast cells and measured radiochromium release. In addition, the antimicrobial activity of 1% neutral buffered Sodium hypochlorite and 0.125% Solvidont against E. faecalis, Escherichia coli, and Candida albicans was measured. From this data, a cytotoxicity-antimicrobial quotient was obtained which indicated that while the antimicrobial activity of Solvidont was superior to sodium hypochlorite, its cytotoxicity was higher. The authors therefore concluded that 0.125% Solvidont “did not provide a better alternative” to 1% sodium hypochlorite.
In all, Salvizol could be used instead of sodium hypochlorite as an irrigation solution because it causes only moderate tissue irritation and has low toxicity when compared to sodium hypochlorite. Salvizol has been widely abandoned in favour of other efficient endodontic disinfectants, because Salvizol was found to have relatively low organic tissue dissolving capabilities when compared to sodium hypochlorite [106]. There appears to be a lack of comprehensive research into Salvizol/Solvidont. Therefore, further studies of Salvizol/Solvidont are necessary for the present day.
3.10. Wescodyne and Iodopax
Wescodyne contains a mixture of non-ionic wetting agents complexed with elemental iodine, which is known for its bactericidal properties. The composition of Wescodyne (West Chemical Products, Inc., Long Island City, NY, USA) is 9% polyethoxy polypropoxy polyethoxy ethanol-iodine complex, 9% nonylphenoxypoly (ethyleneoxy) ethanol-iodine complex, and 82% inert ingredients, which collectively provides a minimum of 1.6% iodine and it is diluted to 0.45% (0.0072% iodine) in distilled water for clinical use [75]. Iodopax (Ferrosan, Sweden) is an aqueous solution of 5% iodine dissolved in octylphenoxy polyglycol ether. It is diluted to 0.8% (0.04% iodine) in distilled water for clinical use [75].
Iodine, like chlorine, binds to the sulfhydryl groups of cysteine in enzymes, causing them to degrade [107]. While iodine is a powerful antiseptic, drawbacks such as hypersensitivity, corrosiveness, and staining have prevented it from being used as an irrigation solution. Organic iodine compounds, or iodophors, such as Iodopax and Wescodyne, on the other hand, are stable vehicles that can leach out the necessary amount of iodine. Iodphors can thus be described as non-staining, having a longer duration of action, and being significantly less irritating than iodine, while still retaining a broad-spectrum antimicrobial action [108]. The iodine vehicles are neutral polymers with surface-active properties that improve the wetting ability of the solutions [109]. In vitro studies revealed a low ratio of antimicrobial to cytotoxic effect when using Iodopax, indicating that the potential for toxicity should determine the concentration of the solution used [93]. Wescodyne had no antimicrobial effect on Staphylococcus pyogenes aureus, E. faecalis, Pseudomonas aeruginosa, or candida, whereas Iodopax acted well against E. faecalis [75]. Both of these compounds were far more cytotoxic to L-cells and HeLa cells than they were bactericidal to the microorganisms tested. Nonetheless, iodophors were considered to be low-surface tension organic iodine solutions that are excellent aids in root canal cleaning without causing allergic reactions. Notably, Wescodyne was acknowledged as a root canal irrigation solution for the last time in 1990, after which it was no longer mentioned in the same context [108].
3.11. Glutaraldehyde and Potentiated Acid 1, 5 Pentanedial
Glutaraldehyde is an antimicrobial agent with a broad spectrum of activity and an immediate effect on viruses, spores, and bacterial cellular components. It is a five-carbon saturated dialdehyde (1, 5 pentanedial) that readily reacts with protein, primarily amino groups, to form stable glutaraldehyde-protein crosslinks. The presence of free aldehyde groups is required for effective biocidal activity. In an acid solution, temperature increases can result in more free aldehyde, whereas in an alkaline solution, temperature increases result in a loss of reactive aldehyde [110, 111]. The biocidal activity of glutaraldehyde is highly dependent on the acidity level, and when the acidity level is higher (pH less than 4), the bactericidal effect is diminished.
On the other hand, the stability of alkaline solutions is much lower than that of acidic solutions because polymerization reactions take place at lower acidity levels, which leads to a loss of antimicrobial activity. The rate of glutaraldehyde polymerization can be slowed down by increasing the level of acidity in the solution, which ultimately results in the solution having a longer shelf life. In clinical settings, glutaraldehyde is typically offered in the form of a 4% solution, which, before application, must first have an “activator” and a “surfactant” added to it to achieve an acidity level of approximately pH 8 and to maintain the antimicrobial effect for longer [111].
Endodontic use of 2% glutaraldehyde as an irrigant that “fixes” necrotic pulp contents was initially proposed by Wemes et al. in 1983 [112]. Because lateral canals could not be accessed during Standard instrumentation, it was hypothesised that glutaraldehyde may be used to fix organic substances irreversibly and instantly. It was stated that because the canal would be quickly sterile due to bacterial fixation, the need for other endodontic medications would be avoided. In a pilot trial of patients having single-appointment endodontics, these authors claimed success rates utilising glutaraldehyde as an irrigant. In vivo, glutaraldehyde was found to be much less irritating to periapical tissues than another aldehyde formulation (formocresol).
In a later investigation, Wemes and Arends (1984) explored the claims of dentine softening with the use of 2% glutaraldehyde (pH 4). Bovine dentine samples were submerged in glutaraldehyde for 10 or 20 minutes before being examined for microhardness differences immediately and 48 hours later [113]. The results showed that dentine softened immediately, followed by an increase in microhardness of up to 15% greater than before. While the authors stated their findings were “significant”, no statistical analysis information was provided. The rise in microhardness after 48 hours was thought to be caused by cross-linking of collagen that had been liberated from mineralised dentine by glutaraldehyde.
Attempts have been made to enhance glutaraldehyde's antibacterial action by including surface-active ingredients. It was hypothesised that this would increase the wettability of bacterial cell walls, allowing for faster drug penetration [114]. Martin (1975) contrasted the bactericidal effects of 2% potentiated acid 1,5 pentanedial, pH 5.8 (Sonacide, Wave Energy Systems, Inc. NY, USA) with 5.5% sodium hypochlorite. Bactericidal activity was demonstrated for both compounds against E. faecalis, Streptococcus Mitis, Staphylococcus aureus, and Escherichia coli. However, in the presence of serum proteins, 5.5% Sodium hypochlorite was shown to be less efficient, and Martin proposed that potentiated acid 1,5 pentanedial has potential as an endodontic irrigant. A later investigation found that when a 2% solution was administered to rabbit eyes and rat connective tissue, it elicited toxic effects, whereas a 1% solution was less harmful [115].
3.12. Potassium Hypochlorite and Hydrogen Peroxide Combination
This combination was first recommended in the early 1970s, according to Schroeder (1981). Potassium hypochlorite or Pulpolyt (Heilmittelwerke, Vienna, Austria) supplied 7% effective chlorine, whereas Perhydrol (Heilmittelwerke, Vienna, Austria) supplied 30% hydrogen peroxide. Pulpolyt, when combined with Perhydrol, is reported to cause micro-explosions within the root canal, which may aid in the cleaning of the canal in a coronal direction [116].
3.13. Decal
Fraser (1974) observed dentine softening after a 15-minute application of Decal (Glover Laboratories, Melbourne, Australia), which included 5.3% oxyacetic acid, 4.6% ammonium oxyacetate, and 0.06% cetyl trimethyl ammonium bromide at pH 3.4. Although Decal softened cervical and mid root dentin to a limited depth, apical dentin was not softened [117]. Decal was tested on specimens from un-instrumented root canal wall surfaces of immature premolars by Koskinen, Meurman, and Stenvall (1980). Decal had little effect on organic tissue, although it did cause decalcification of exposed mineralised dentine [101].
3.14. Polyacrylic Acid
McComb and Smith (1975) used scanning electron microscopy to demonstrate that 20% polyacrylic acid irrigation was inferior to EDTA on instrumented root canal surfaces. This was believed to be due to polyacrylic acid's increased viscosity and consequently slower flow [118]. A second scanning electron microscopy investigation looked at less viscous polyacrylic acid solutions (5% and 10%), but the results were disappointing. The solutions etched accessible portions of the canal and removed the smear layer, but more apically, the solution tended to coat the canal [119].
3.15. Nelex
The efficiency of the irrigant Nelex (BYK-Gulden, Lomberg, GMBH, Konstanz, West Germany), a condensate of m-cresolsulphonic acid, and formaldehyde (pH 0.6), was examined by Hakala, Koskinen, and Narvainen (1976). The authors reported that Nelex was well tolerated by tissues, with a preference for selectively dissolving necrotic tissue while leaving normal tissue unaffected.
It was also claimed that, whereas inorganic material dissolving property was pH dependent, organic necrotic matter dissolution was concentration dependent [120]. In the demineralisation of powdered dentine, Nelex was found to be seven times more effective than EDTA [101]. However, the authors noted that Nelex's strong acidic impact could erode enamel and render other medications ineffective. A subsequent investigation discovered that 5% and 20% Nelex had a strong demineralising impact on un-instrumented root canal dentine, which extended into the tubules and resulted in tubule orifice funnelling.
3.16. Hydrogen Peroxide
Hydrogen peroxide is an oxidizing agent that is rapidly decomposed by tissue catalase into molecular oxygen and water [51]. At the beginning of the 20th century, hydrogen peroxide was considered an antiseptic of only marginal value [31]. This was due to the fact that it was only considered for the mechanical detergent action connected with the rapid disengagement of oxygen gas on infected surfaces. This was thought to be of greater value than any antiseptic action that the hydrogen peroxide itself might have. However, just recently, it has been reported that hydroxyl radicals could be generated from hydrogen peroxide through the use of ultrasound and that this process had a bactericidal effect against E. faecalis as well as the potential to be usefully applied in the disinfection of root canals [121, 122].
Researchers' views on hydrogen peroxide's utility evolved over time, from having little to no value to being of intense interest. Hydrogen peroxide cannot dissolve necrotic tissue or organic debris on its own [51, 123], and its activity was found to be brief and ineffective [51]. It was also thought that using hydrogen peroxide was risky due to the possibility of oxygen release into the periapical tissues [124]. The use of hydrogen peroxide, on the other hand, was encouraged, with the idea that its foaming action would force debris out of the canal [125]. Weine (1982) recommended hydrogen peroxide as the irrigant of choice for perforations involving the apical constriction area, claiming that the release of oxygen from hydrogen peroxide in contact with a tissue would kill strict anaerobes during endodontic procedures [56]. Hydrogen peroxide has traditionally been used at a concentration of 3% in conjunction with sodium hypochlorite [71].
3.17. Hydrogen Peroxide and Sodium Hypochlorite - A Combination that was Never Meant To Be
In 1943, Grossman proposed using double-strength sodium hypochlorite “chlorinated soda” in conjunction with 3% hydrogen peroxide [32]. The effervescence produced by combining the two solutions was thought to liberate newly formed oxygen, which could then follow the path of least resistance toward the canal's opening. Grossman recommended using double-strength sodium hypochlorite (a reducing solution) as the final irrigant to avoid gas pressure buildup from any remaining hydrogen peroxide (an oxidising solution).
From the 1940s to the 1960s, several authors supported the combination [63, 126-128]. Sommer, Ostrander, and Crowley (1966) thought hydrogen peroxide was “useful” in large canals [129]. Stewart (1955) advocated for an irrigation technique that used 0.5 ml of 3% hydrogen peroxide followed by 0.5 ml of sodium hypochlorite after each instrument change, claiming that his results showed that 94% of canals were rendered sterile after this procedure [126]. Morris (1958) recommended alternate irrigation with hot sodium hypochlorite and room-temperature hydrogen peroxide, followed by sodium hypochlorite [127]. During the treatment of teeth with non-vital pulps, the use of 6% hydrogen peroxide and double-strength sodium hypochlorite was advised by Maine (1959) [63].
Marshall, Massler, and Dute (1960) reported increased radicular dentine permeability after root canal treatment with 3% hydrogen peroxide and 5.25% sodium hypochlorite. The combination proved to be more effective than either solution alone [130]. Svec and Harrison (1977) observed using a light microscope that a combination of 5.25% sodium hypochlorite and 3% hydrogen peroxide was more effective than saline in debridement of the canal's apical 3 mm. There was no significant difference between the control and experimental groups at the 5 mm level [33].
Later studies compared the mixture to sodium hypochlorite alone. At the apical 3 mm of the canal, no significant difference was found between the two groups. The authors concluded that the effervescence which occurs when sodium hypochlorite is combined with hydrogen peroxide “may not offer a decided advantage in terms of increased debridement of the apical portions of root canals in single-rooted teeth” [131]. Furthermore, Harrison and Hand (1981) reported that 3% hydrogen peroxide plus 5.25% sodium hypochlorite had no antibacterial effect in vitro against E. faecalis, whereas 5.25% sodium hypochlorite alone was effective in 45 seconds [132]. Other research has found that combining hydrogen peroxide and sodium hypochlorite does not provide superior irrigating properties [55, 61, 133-135].
Thé (1979) reported that a combination of 2% or 5% sodium hypochlorite and 3% hydrogen peroxide was less effective for dissolving necrotic tissue than sodium hypochlorite alone [133]. Furthermore, when hydrogen peroxide was added to the solution, the solvent action of sodium hypochlorite was likely to be reduced. According to Thé, there was no proof for the assumption that the release of newly formed oxygen of hydrogen peroxide can bubble out debris from the root canal [133]. Brown and Doran (1975) reported that when the combination was used within 2 mm of the debris, dentine particles in simulated canals were “agitated,” but this was shortly followed by particle settling [55]. The use of sodium hypochlorite and hydrogen peroxide in an alternating fashion, as reported by Baumgartner and Ibay (1987), appears to be of no benefit [136].
3.18. Calcium Hydroxide
A biocompatible irrigation solution that has been suggested is a calcium hydroxide solution at a 10% concentration [137]. For many years, endodontists have relied on calcium hydroxide as an intracanal medication due to its antibacterial effects, which result from the high pH (approximately 12.5), which disrupts cell membranes and protein structure. The low water solubility and restricted diffusion of calcium hydroxide contribute to its biocompatibility by limiting its cytotoxicity to the contact tissues. It is challenging to rapidly reach and sustain the high pH necessary for effective antimicrobial action against E. faecalis sequestered in dentinal tubules due to the low solubility and diffusion of calcium hydroxide and the buffering capacity of dentin. Calcium hydroxide was effective in cases where gram-negative bacteria were the predominant cause of periapical lesions because it can degenerate endotoxins, which are a component of the cellular membrane. However, calcium hydroxide takes a considerable amount of time to break down and dissolve the necrotic tissue remnant and bacterial byproducts [138], which limits its usage as a root canal irrigation solution. One study found that combining chlorhexidine and calcium hydroxide resulted in significantly less E. faecalis growth than chlorhexidine alone, especially when the combined solution was heated [139].
3.19. Punica Granatum
Punica granatum is a pomegranate extract obtained from the peel. Although it has only recently attracted researchers as a potential irrigation solution [140], it has a long history in medicine due to its constituent ingredients, flavonoids, and tannins, as well as other phenolic compounds, which have bactericidal, antifungal, antiviral, immune modulation, and anthelminthic properties [141]. Antimicrobial activity of 20% Punica granatum against E. faecalis was evaluated, and the combination of punica granatum with sodium hypochlorite and chlorohexidine was found to be more effective than either irrigant alone or a mixture of sodium hypochlorite and chlorohexidine. This observation was attributed to the presence of ellagitannin and punicalagin, which can obstruct microorganism adhesion to tooth surfaces by interfering with the polyglycan synthesis process [142]. Furthermore, combining Punica granatum with either sodium hypochlorite or chlorhexidine improved antimicrobial activity against E. faecalis in both cases, but the effect was stronger when combined with chlorhexidine [143].
3.20. Medium-chain Fatty Acids
Medium-chain fatty acids were reported to be bactericidal against Gram-positive bacteria as far back as the 19th century [144]. According to the findings of one study, medium-chain fatty acids exhibited an inhibitory action against E. faecalis [145]. This action can be linked to the medium-chain fatty acids' surfactant activity as well as their ability to disrupt the cell membrane, which ultimately leads to cellular lysis. Among the medium-chain fatty acids that were evaluated (i.e., lauric acid, decanoic acid, and octanoic acid), lauric acid showed the greatest potential to inhibit E. faecalis growth [145]. Although medium-chain fatty acids have the potential to be used in root canal therapy and incorporated into irrigation solutions, more research is required before they can be used in clinical practice.
3.21. Chitosan
Chitosan is a natural polysaccharide derived from the shells of crustaceans that are composed of glucosamine co-polymers and N-acetylglucosamine [146]. It has the advantage of being nontoxic, biocompatible, and biodegradable. Because of its chelating effect, broad-spectrum antimicrobial activity, and ability to inhibit E. faecalis intercellular and intracellular activities, chitosan has been proposed for use as an endodontic irrigation solution [147].
It was shown that the polycationic/polyanionic nature, high surface area, and charge density of chitosan nanoparticles can collectively contribute to chitosan’s antimicrobial effect [148, 149]. Using chitosan as an irrigation solution at a concentration of 3% makes it as effective against E. faecalis as sodium hypochlorite [150]. Another study found that chitosan has a high bactericidal effect against both gram-negative and gram-positive bacteria [151]. In a study that compared the antimicrobial effects of various irrigation solutions, chitosan was found to be less effective than chlorhexidine and sodium hypochlorite against E. faecalis [152]. Chitosan paste reduced the viability of E. faecalis and C. albicans in a root-canal biofilm model when used as an intracanal medication for 7 days, suggesting that it could be used as an intercanal medicament rather than an irrigation solution [153]. In either case, if further research is conducted to determine the optimal formulation and dosage, it may be an effective adjunctive agent for retreatment cases.
3.22. Nigella Sativa, Azadirachta Indica and Aloe Vera
Nigella sativa, Azadirachta indica, and Aloe vera are some of the more recent plant-based compounds studied for use as potential root canal irrigation solutions [154]. Nigella sativa, also known as Black Seed or Black Cumin, is a plant in the Ranunculaceae family that has been used to treat illnesses such as asthma and bronchitis [155]. Aqueous Nigella sativa inhibits candidiasis activity in mice [156]. The antibacterial effect of Nigella sativa seeds against various types of microorganisms has also been demonstrated [157]. Azadirachta indica (neem) is a popular and widely available herb that has been used as a remedy for many years and has been suggested to be used against E. faecalis, S. mutans, and C. albicans due to its antimicrobial activity [158].
An in vitro study was carried out to evaluate the antimicrobial efficacy of aqueous Nigella sativa and Azadirachta indica against E. faecalis, P. aeruginosa, and C. albicans. Both aqueous extracts demonstrated improved antimicrobial activity against all tested species [155]. In comparison to other herbal extracts, the antimicrobial efficacy of Azadirachta indica as an irrigant against E. faecalis and Candida albicans was superior to sodium hypochlorite [159]. There was no significant variation in antimicrobial efficiency against E. faecalis between Aqueous Nigella sativa, Azadirachta indica, sodium hypochlorite, and chlorhexidine, with the exception of Azadirachta indica, which demonstrated some growth of E. faecalis colonies. The long-term efficacy of Azadirachta indica as a disinfectant against E. faecalis is still being studied [160].
Aloe vera is environmentally friendly and inexpensive, and it may have antibacterial, anti-inflammatory, antifungal, and analgesic properties (Subapriya and Nagini, 2005). Aloe vera can exhibit antimicrobial activity against E. coli, E. faecalis, and S. aureus [160-162]. A study comparing the antibacterial effects of Aloe vera as an irrigation solution in root canal treatment to sodium hypochlorite and chlorhexidine found comparable antibacterial effects against E. faecalis [163, 164].
3.23. QMix
QMix is a combination of different substances, including 2% chlorhexidine, 17% EDTA, saline, and cetrimide. This material was first introduced as an experimental antibacterial root canal irrigation solution by Dai et al. in 2011 [165]. QMix demonstrated a high capability of removing the smear layer and eradicating E. faecalis, high wettability, favorable surface tension properties, and biocompatibility, which, in several reports, demonstrated superior antimicrobial effects when compared in vitro to sodium hypochlorite and chlorhexidine [165-167]. In a recent randomized clinical trial, however, the use of QMix irrigation solution as a final rinse resulted in no significant difference in periapical healing compared to sodium hypochlorite alone at the end of a one-year follow-up period [168].
3.24. HybenX
Sulfonated phenolics make up sixty percent of the total volume of the novel hygroscopic solution known as HybenX, and the remaining forty percent is made up of sulfuric acid and water. In 2013, the Food and Drug Administration approved this desiccating agent to be used as an adjunctive rinse of tooth root canal systems to improve the removal of the smear layer from within the root canal spaces. The solution demonstrated promising anti-biofilm activity [169]. Recent in vitro research has shown that this compound is effective in inhibiting the growth of E. faecalis [170, 171]. HybenX was found to be effective at reducing E. faecalis biofilm and removing smear layers from root canal systems, making it a promising irrigating agent in infected root canals [172].
3.25. Octenidine
Octenidine hydrochloride is a cationic surfactant derived from pyridine [173]. It is a new bispyridine antimicrobial compound that has been developed as an antimicrobial agent for use in mouthwash formulations. It has been shown to be an antiseptic for mucous membranes and is also used in severe burns and to promote wound healing. Stadler, Bogiatzis, and Gehrer proposed octenisept as an endodontic irrigant in 1998 due to its antimicrobial properties and low cytotoxicity [174].
Octenidine hydrochloride is a stable compound that has bactericidal and fungicidal activity by altering the membranes and walls of cells [175]. It is thought to have more antibacterial properties than chlorhexidine [175-178]. It was also introduced as a mouthwash at 0.1 percent concentration because it contains a bispyridine derivative that can reduce plaque accumulation and thus gingivitis [179].
In comparison to chlorhexidine and sodium hypochlorite, it appears that octenidine has a lot to offer as a potent irrigation solution, especially as a substitute in patients who are allergic to sodium hypochlorite [173]. One study looked at the ability of three different intracanal irrigation solutions— chlorhexidine, sodium hypochlorite, and octenidine—to eradicate E. faecalis inside the root canal. Octenidine was the most effective in eliminating E. faecalis from the canals, followed by sodium hypochlorite and chlorhexidine [180].
In contrast, a recent study discovered that octenidine had greater antimicrobial activity than green tea, but both were less effective than chlorhexidine and sodium hypochlorite. However, it was suggested that octenidine irrigation solution be used in conjunction with sodium hypochlorite to mitigate its disadvantages and enhance its antimicrobial properties [181]. More research will be required to determine the best formulations and dosages of Octenidine hydrochloride.
3.26. Sodium Hypochlorite
Sodium hypochlorite's antimicrobial activity and ability to dissolve organic tissues make it the most popular choice for use in endodontic treatment [37]. However, there is disagreement about the optimal sodium hypochlorite concentration, which can vary from 0.5% to 5.25%. Moreover, since sodium hypochlorite can irritate periapical tissues, it's preferable to look for alternative root canal irrigation solutions that are less likely to cause unwanted side effects [182].
The English chemist Henry Drysdale Dakin and the French surgeon Alexis Carrel (1915) are credited with introducing sodium hypochlorite into medicine when they reported the use of a 0.5%-0.6% Sodium hypochlorite solution “Dakin's solution or Carrel-Dakin fluid” for the irrigation of soldiers' wounds during World War I [31]. To make this solution, dissolve sodium carbonate in tap water and add chlorinated lime. Boric acid was then added to the filtrate, yielding a one-week shelf-life solution. Because of the potential for tissue irritation, too much free alkali was to be avoided. Dakin's solution was a British Pharmacopoeia standard product for more than 50 years, until 1968 [183].
Crane is credited with the first use of sodium hypochlorite in endodontics in 1920 [184, 185]. Walker recounted his clinical observations after using a double-strength chlorinated soda solution during endodontic treatment, a regimen allegedly suggested by Dr. Blass of New York University, in 1936 [186]. Walker described this solution as a powerful germicide as well as a “very satisfactory” organic solvent based on his clinical experience. Grossman and Meiman later confirmed the solvent properties of a double-strength chlorinated soda solution (1941) [30].
Both Stewart (1955) and Masterton (1965) claimed that chlorinated soda provided good post-operative results in the removal of necrotic tissue from the root canal [124, 126]. When Sommer, Ostrander, and Crowley (1966) recommended the use of 4%-6% sodium hypochlorite with tincture of green soap [129], and when Newman (1983) maintained that freshly made electrolytic sodium hypochlorite was the ideal solution for endodontic therapy, there was a massive surge in the popularity of the solution that followed [187].
Sodium hypochlorite solution is one of several preparations (known as chlorophers) that produce hypochlorous acid from chlorine. These agents are effective antimicrobial and bleaching agents, and they dissolve blood clots and necrotic debris with ease. Chlorine acts both as an element and as undissociated hypochlorous acid, which is formed by the hydrolysis of chlorine. The ease and degree of hypochlorous acid liberation of a chloropher is directly related to its antimicrobial efficacy [51].
An indicator of oxidizing capacity, “available chlorine” (as defined by Penick and Osetek, 1970) is expressed as a percentage, and a 5% sodium hypochlorite solution produces around 1% available chlorine [51]. Because of its strong oxidizing properties, chlorine is a strong electron-attracting agent. The reduction of chlorine to chloride diminishes its disinfectant properties. It takes little time for chlorine to react with inorganic substances, but the rate at which it can react with organic compounds varies depending on how much chlorine is on hand [183]. Sodium hypochlorite reactions are not typically dependent on hydrogen bonding and van der Waals forces, but rather on covalent bonding or the formation of strong ionic attachments [188].
To a limited extent, chlorine's bactericidal activity can be impeded by organic debris, which binds to the element and prevents it from forming hypochlorous acid [51]. Thus, it has been emphasized that “the full germicidal potential of Sodium hypochlorite cannot be realized until thorough debridement has been accomplished” [51]. The antimicrobial activity of sodium hypochlorite was found to be diminished after it reacted with proteins and other cellular debris, as reported by Cotter et al. (1985). Thus, further applications of sodium hypochlorite were required to maintain effective antimicrobial coverage [189].
Knox et al. (1948) described chlorine's antimicrobial action as resulting from the oxidation of sulfhydryl enzymes required for glycolysis [190]. Spangberg (1973) defined this action as the oxidation of cysteine-containing enzymatic proteins (which has a sidechain terminating in a sulfhydryl group) [75]. Because these enzymes can only function if the sulfhydryl group is reduced and free, the effective binding of these sulfhydryl groups by halogens like chlorine is extremely damaging to the cell. Chlorine can also oxidize the sulfhydryl groups of coenzyme A (an acyl group carrier) [75].
The concentration of undissociated hypochlorous acid, and thus the antimicrobial activity of chlorine in solution, is greatly influenced by the acidity level (pH). Lowering the pH increases sodium hypochlorite's biocidal activity [191], while raising the pH significantly decreases this activity [183]. Sodium hypochlorite solutions, on the other hand, are less stable when formulated at a lower pH [189].
Pashley et al. (1985) described a 5.25% solution of sodium hypochlorite as containing approximately 5% sodium chloride in a very alkaline (pH 11) solution of sodium hydroxide, making the solution very hypertonic (around 2800 mOsmol/kg) [192]. These authors maintained that the solution's clinical efficacy was due to its ability to oxidize, hydrolyze, and, to some extent, osmotically draw fluids out of tissues.
Lewis introduced a commercial source of sodium hypochlorite in 1954 under the brand name Clorox (The Clorox Company, Oakland, California, USA) [193]. Clorox (pH 11.0-11.5) was analyzed by Shih, Marshall, and Rosen (1970) and found to have 5.25% sodium hypochlorite, 0.20% sodium carbonate, 4% sodium chloride, and 0.005-0.01% free sodium hydroxide [194].
Earlier dental literature contains references to “Zonite” (Zonite Products Corporation, New York City, NY) (51), a (then) commercially available 5% sodium hypochlorite solution, as well as the more recent “Milton” solution (Richardson-Vicks Ltd, Eghan, Surrey, England), which contains 1-2% (w/v) sodium hypochlorite and 16.5% (w/v) sodium chloride [195, 196]. Newman (1983) questioned the use of 2% Milton solution after observing a high degree of crystalline encrustation in the dental suction unit following its use [187].
Cameron (1988) detailed the processes used to produce sodium hypochlorite in the 1980s and reported that the varying strengths of commercially available sodium hypochlorite solutions are the result of differences in the stock solution's dilution, buffering, stabilization, and packaging [197]. Interestingly, disagreement has persisted about the optimum concentration of sodium hypochlorite until the time of this manuscript's completion [182].
Historically, and until now, the stock solution was prepared by passing chlorine gas through a column of sodium hydroxide, yielding a solution containing 12.5% available chlorine and 0.1% to 0.5% sodium hydroxide:
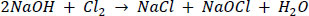 |
The presence of sodium hydroxide raises the PH to 12, increasing the solution's stability. The pH of a solution can be reduced by diluting it or adding sodium chloride. At low concentrations, increases in stability have an exponential value, with the shelf life of 12%, 5%, and 3% sodium hypochlorite solutions being approximately one, four, and ten years, respectively. During the decomposition of sodium hypochlorite, two reactions occur, the second of which accounts for 85% of the decomposition reaction [197]:
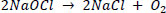 |
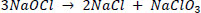 |
Grossman (1943) claimed that storing double-strength chlorinated soda solution in a cool place would allow for a three-month shelf life [32], and Moorer and Wesselink (1982) recommended brown glass bottles for at least one month of stability (198). Velvart (1987) reported that the concentration of test solutions of 0.5%, 1%, and 2% sodium hypochlorite did not change over a two to six-month observation period when stored in dark brown bottles at either 4°C or room temperature [199]. A diluted Milton solution was found to be stable at room temperature for at least a year [200].
Cunningham and Balekjian (1980) investigated the stability of sodium hypochlorite under 37°C conditions [184]. After 24 hours, the available chlorine content of 5% and 2.5% sodium hypochlorite decreased by 9.5% and 4%, respectively. Clarkson, Moule, and Podlich (2001) emphasized the importance of storing sodium hypochlorite in closed, opaque containers. They found that constant container opening caused a greater loss of chlorine concentration in diluted bleach solutions, possibly because a lower concentration of sodium hydroxide allows the pH to drop more quickly.
Although sodium hypochlorite is by far the most popular irrigant, it is not without flaws: as stated earlier, the solution’s free chlorine works by converting proteins in the pulp tissue into amino acids, and this reduces the solution’s concentration, resulting in a reduction in its toxicity, antibacterial ability, and tissue dissolving capability. Furthermore, it has an unpleasant odor and taste, and it can cause severe tissue damage, and toxicity when it inadvertently penetrates the periradicular tissues [179].
3.27. Chlorohexidine
After extensive testing, chlorhexidine was created in the late 1940s by researchers at Imperial Chemical Industries Ltd. (Macclesfield, England). At first, anti-viral substances were synthesized by synthesizing a series of poly-bisguanides. Although they showed limited effectiveness against viruses, they were eventually rediscovered as potent antimicrobial agents. Among the bisguanides tested, chlorhexidine was the most effective [201]. Chlorhexidine is a strong base that is most stable in salt form. The original salts were chlorhexidine acetate and hydrochloride, which are both relatively water-insoluble. As a result, chlorhexidine digluconate has taken its place [202].
Chlorhexidine di-/gluconate is a 20% w/v aqueous solution that is used to make several formulations containing 0.02%-1.0% w/v chlorhexidine [110]. The gluconate solution is used in alcohol solutions ranging in concentration from 60% to 90%. The majority of chlorhexidine research for dental use has focused on its role in plaque control [203]. However, at the Adelaide Dental School in 1962, Savion was introduced as an irrigant, either alone or as an adjunct to EDTA. The solution Savion (ICI, Australia) contains 0.03% chlorhexidine and 0.3% cetrimide [204].
Some anions, such as natural soap, synthetic detergents, and inorganic anions like chloride and phosphate, are incompatible with chlorhexidine [110]. The optimal pH for chlorhexidine antimicrobial activity is 5.5 to 7.0. Above pH 8.0, chlorhexidine base precipitates, and at low pH, the solution's activity gradually declines [110]. At room temperature, chlorhexidine was reported to be very effective against vegetative gram-positive and gram-negative bacteria. It is also said to have a broad-spectrum antibacterial action, with greater activity against gram-positive microorganisms than gram-negative microorganisms [203]. Emilson (1977) reported the effectiveness of chlorhexidine against a variety of aerobic, facultative anaerobic, and anaerobic microorganisms in vitro [205]. Other in vitro studies have revealed that chlorhexidine can be adsorbed to hydroxyapatite and that when the concentration of chlorhexidine in the immediate environment is low, the adsorbed chlorhexidine is released [205-207].
Inadvertent intravenous administration of up to one-liter of 1 in 5000 (0.02% w/v) chlorhexidine resulted in haemolysis due to the resulting hypotonicity rather than a direct effect of the chlorhexidine, and patient recovery was complete after exchange transfusion. Concentrations greater than 2% may cause dermal discomfort, while concentrations as low as 0.2% are tolerated by the eye. Spangberg, Engstrom, and Langeland (1973) discovered that 0.05% chlorhexidine was ten times more toxic to L-cells and HeLa cells than it was bactericidal. In vitro and in vivo testing of 0.2% chlorhexidine revealed that it was less toxic to HeLa cells and rabbit muscle tissue than 5% chloramine-T and had a similar effect to Biosept, Iodopax, and 0.5% Sodium hypochlorite (Hibitane (Imperial Chemical Industries, England) contains 0.2% chlorhexidine).
In the 1970s, studies assessing the reaction of various body tissues to chlorhexidine started to appear in the published literature. Foulkes (1973) reported that the inadvertent intravenous administration of up to one liter of 1 in 5000 (0.02% w/v) chlorhexidine resulted in haemolysis from the ensuing hypotonicity rather than from a direct effect of the chlorhexidine, and that the patient recovered completely after receiving an exchange transfusion [202]. Chlorhexidine concentrations of less than 2% are well tolerated by the skin, and concentrations of less than 0.2% are tolerated by the eye [110]. Spangberg, Engstrom, and Langeland (1973) discovered that 0.05% chlorhexidine was ten times more toxic to L-cells and HeLa cells than it was antimicrobial [75]. In vitro and in vivo testing of 0.2% chlorhexidine revealed that it was less toxic to HeLa cells and rabbit muscle tissue than 5% chloramine-T and had a similar effect to Biosept, Iodopax, and 0.5% Sodium hypochlorite [74, 208].
Tucker, Mizrahi, and Seltzer investigated the efficacy of a 0.1% chlorhexidine solution using SEM (1976) [209]. Their findings were compared to those of Baker et al. (1975), who conducted another SEM study in which many irrigation solutions were evaluated [210]. In both studies and regardless of the irrigant used, the results showed that no canal was free of debris. Parsons et al. (1980) investigated the adsorption of 0.2% and 1% chlorhexidine to bovine pulp and dentine specimens after 20- and 40-minute contact times, respectively. Chlorhexidine release from specimens was measured either immediately or one week later. Antibacterial activity against E. faecalis was also evaluated. It was reported that both pulp and dentine specimens absorbed and released chlorhexidine. Furthermore, antimicrobial properties were acquired and remained unchanged one week later. Changes in concentration and duration of exposure did not affect chlorhexidine uptake or the acquisition of antimicrobial properties by the specimens [208].
While chlorhexidine appears to be a better irrigant than saline [211], it does not appear to be as effective as 2.5% sodium hypochlorite [212]. Delaney et al. (1982) compared the antimicrobial effect of 0.2% chlorhexidine to 0.9% sterile saline in vitro. Although the results showed that chlorhexidine significantly reduced the number of viable microorganisms, the experimental methodology suggested that the use of an intracanal medicament may have influenced the results [211]. Ringel et al. (1982) conducted an in vivo study comparing 0.2% chlorhexidine to 2.5% sodium hypochlorite. Anaerobic culturing of necrotic pulps in 60 teeth with positive cultures at the start of treatment revealed that 2.5% sodium hypochlorite was a more effective antibacterial agent than 0.2% chlorhexidine. The hypothesis that chlorhexidine would be a better agent over time due to its ability to be adsorbed and released by dental tissues, resulting in a longer effect, was not supported. Over three appointments, the prevalence of positive cultures and culture reversals indicated that chlorhexidine did not have a long-lasting bactericidal effect [212]. This and similar claims were refuted by White, Hays, and Janer (1997), who reported that chlorhexidine has long-lasting residual antimicrobial activity when used as an endodontic irrigant [213]. The long-lasting residual activity was confirmed by several other works published afterward [214, 215].
A recent meta-analysis of randomized controlled trials and a systematic review looked at the incidence of bacterial proliferation and the variation in bacterial number in root canals after irrigation with different solutions and found no significant differences in the incidence of positive bacterial proliferation between groups treated with chlorhexidine and sodium hypochlorite [216]. When used as root canal irrigation solutions, chlorhexidine, and sodium hypochlorite are effective antimicrobial agents. The two chemical agents have the same ability to reduce bacterial loads in the canals. However, gram-negative bacteria survived, indicating that neither irrigation solution is capable of eliminating bacteria.
Clinicians and researchers alike have been tempted to include chlorhexidine gluconate in the root canal treatment regimen at least as an adjunctive irrigation solution because of its proven efficacy as an effective irrigation solution with broad antibacterial activity, low toxicity, and no malodor or unpleasant taste [217, 218]. Initially, mixing sodium hypochlorite with chlorhexidine was thought to be beneficial because the mixture could reach deeper parts of the dentinal tubules. Because chlorhexidine has synergistic antimicrobial effects in endodontic infections, the combination of sodium hypochlorite and chlorhexidine was found to boost antimicrobial efficacy [37]. Several reports, however, were critical of the concept due to the formation of an orange-brown precipitate when mixing sodium hypochlorite and chlorhexidine [219]. This precipitate strongly adheres to the canal walls resulting in their discoloration and compromising the seal [37, 219]. Nonetheless, the mixture results in less postoperative pain than either of the solutions alone [219]. Chlorhexidine gluconate is now being considered a promising irrigation agent to replace sodium hypochlorite during root canal disinfection and endodontic instrumentation. Chlorhexidine's antibacterial properties have been extensively demonstrated when used as an adjunct treatment for various oral diseases. The inability of chlorhexidine to dissolve pulp tissue is its main limitation as an endodontic irrigant [182].
Alexidine is a bisbiguanide with a similar composition to chlorhexidine, but it has a longer substantivity and a comparable, if not greater, antibacterial effect on E. faecalis. It can be used either alone or in conjunction with sodium hypochlorite. According to a study that compared the antibacterial activity of alexidine alone or in combination with sodium hypochlorite and chlorhexidine as a final irrigant, the results show that 1% alexidine alone is not as effective as when combined with sodium hypochlorite and chlorhexidine [220]. More importantly, unlike chlorhexidine, the color of the solution remained clear after mixing with sodium hypochlorite, and no precipitate was observed even after centrifugation [221]. It is important to note that the increased affinity of alexidine for bacterial lipopolysaccharide and lipoteichoic acid, two of the major virulence factors in the structure of E. faecalis, is the primary mechanism by which this antibiotic inhibits bacterial growth [222].
3.28. Sodium Hypochlorite and Chlorhexidine: Another Combination that was Never Meant To Be
The two irrigants that are utilized most frequently in the process of root canal treatment are sodium hypochlorite and chlorhexidine. It is common knowledge that sodium hypochlorite can kill microorganisms and dissolve organic tissue. In addition to its broad antimicrobial activity, chlorhexidine also offers substantivity, which is not a property that sodium hypochlorite possesses. Chlorhexidine, on the other hand, does not possess the tissue-dissolving capability that sodium hypochlorite does [213]. As a consequence, the idea of putting them both on the same irrigation regimen came up.
The concept of combining different irrigation solutions came about as a result of the realization that none of the currently available solutions possesses all of the qualities that would make them ideal on their own. Although the combination of certain solutions, and in particular sodium hypochlorite and chlorhexidine, showed some promising synergistic effects, it resulted in the formation of orange-brown precipitation that is not welcome. Initially, the precipitant was thought to be para-chloroaniline, a known toxicant [167]. However, chemical analyses using spectroscopy and chromatography revealed that the precipitate does not contain para-chloroaniline [223, 224]. As a result, the exact chemical composition of the precipitant remains unknown.
This precipitate is the result of an acid-base reaction that takes place after the two substances are combined. Para-chloroaniline has been reported to be cytotoxic, and it has the potential to cause dentinal discoloration and obstruction of dentinal tubules [225]. Additionally, it has the potential to interfere with root canal-filling materials [167, 219]. Because of the insoluble nature of the unwanted precipitate and the yet-to-be-confirmed potential for carcinogenicity, there is now evidence against using chlorhexidine and sodium hypochlorite irrigation solutions together, or even one after the other, during root canal treatment [225].
It is worth noting that alexidine is being proposed to replace chlorhexidine as an adjunct to sodium hypochlorite, a once-highly recommended combination that hasn't lasted as long as expected due to the reported precipitate that proved to be problematic to the treatment's success and the patient's overall health [221]. This is due primarily to the fact that the interaction of alexidine and sodium hypochlorite does not result in precipitates and that alexidine has superior antimicrobial activity. As a result of these factors, alexidine is a more effective and safer alternative to chlorhexidine as an adjunctive endodontic irrigation solution.
Interestingly, several aliphatic amines, as well as other unidentified compounds, were identified as byproducts of the reaction of alexidine and sodium hypochlorite in a recent report [226], Aliphatic amines have previously been shown to be cytotoxic, and the neurotoxicity of the other compounds is suspected due to structural similarities to known neurotoxins [227].
It is hoped that the entire series of bisbiguanides and chlorhexidine analogues will be tested to determine the best substitute for chlorhexidine as an adjunct to sodium hypochlorite, as their antimicrobial activity against gram-negative and gram-positive oral bacteria has previously been confirmed in vitro but still needs to be tested as an irrigation solution in root canal treatment [228]. Along with chlorhexidine and alexidine, bisbiguanide analogues and lab-prepared modifications include isononabutidine, nonabutidine, octihexidine, heptoctidine, hexidecidine, heptihexidine, hexoctidine, and hexhexidine. It is important to note that while most of the investigated bisbiguanides showed broad antimicrobial activity, hexidecidine and hexhexidine had the most promising potential, with activity equal to that of chlorhexidine when tested against the entire range of organisms [228].
Many potential structural modifications can be made to bisbiguanides and chlorhexidine analogues to improve their activity against oral microorganisms, particularly E. faecalis. Because structural variations in bisbiguanides affect activity differently among the various bacterial species associated with dental diseases, attention should be focused not only on the specific therapeutic benefit desired from such an agent but also on the expected reaction with sodium hypochlorite when optimizing bisbiguanide structure. Before suggesting one bisbiguanide analogue as a potential adjunct, all possible byproducts must be carefully plotted to avoid the unintended consequence of being listed under the contraindicated combinations, as was the case with chlorhexidine when combined with sodium hypochlorite, and it appears that alexidine will follow suit. This will only be possible if researchers, including chemists, microbiologists, and dentists, all work together in a multidisciplinary manner.
CONCLUSION
The optimal goal of root canal treatment is to eradicate microorganisms within the root canal system, which in turn would reduce the probability of re-infection and treatment failure. Since complete removal is impossible given the now-available irrigation solutions, clinicians should aim at reducing the number of microorganisms that are present in root canal spaces to a level where their presence would not pose any negative effect on the treatment outcome.
The lessons learned from this historical overview are two-fold: the first is that sodium hypochlorite has established its place as one of the primary ingredients in the development of the optimum combination of irrigation solutions, and the other ingredient should be able to compensate for the sole property sodium hypochlorite lacks substantivity. The second is that the search for an ideal solution to perform all of the functions expected of an irrigation solution has waned, and the focus is now on identifying the best solution to serve as an adjunct to sodium hypochlorite. It follows that this can only be obtained by experimenting with a compound, either as-is or in an altered form, that belongs to the family of compounds that best exhibits the substantivity property: bis-biguanides.
This study suggests further research on two fronts: the first is to identify the ideal companion to sodium hypochlorite that yields no undesirable reaction precipitates, and the second is to keep the effort going toward the formulation of an ideal single irrigation solution that can adequately disinfect the canal while posing no risks to the vital tissues. These recommendations should be considered because historically, the best irrigation formulas were initially opposed by some and were only fully implemented after a significant number of research studies were conducted.
LIST OF ABBREVIATIONS
| EDTA | = Ethylenediaminetetraacetic Acid |
| SEM | = Scanning Electron Microscope |
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


