Effectiveness of Mouthwash-Containing Silver Nanoparticles on Cariogenic Microorganisms, Plaque Index, and Salivary pH in A Group of Saudi Children
Abstract
Objectives:
To compare the effectiveness of Silver nanoparticles (AgNPs) and Chlorhexidine (CHX) mouthwash on Streptococcus mutans (S. mutans), Lactobacillus spp., and Candida albicans (C. albicans) counts O’Leary plaque index (O’Leary PI) scores, and salivary pH levels among children.
Materials and Methods:
The study sample consisted of 117 eligible participants aged 12–18 years. They were divided into control, CHX, and AgNPs groups, with 39 subjects per group. The log10 salivary microbial counts, O’Leary PI, and salivary pH values were recorded and statistically analyzed at baseline and the 28th day. Descriptive statistics were presented as the mean ± standard deviation. In addition, the analyses of variance (ANOVA) and Tukey posthoc test were implemented. The p-value ≤ 0.05 denotes a significant difference between the two points.
Results:
AgNPs and CHX mouthwash were found to have significantly reduced salivary microbial counts and O’Leary PI scores. The salivary pH levels notably increased on the 28th day (p-value < 0.001). All measured outcomes demonstrated notable effects, with the greatest observed for the CHX group, followed by the AgNPs group, and finally, the control group.
Conclusion:
Chlorhexidine and AgNPs mouthwash effectively reduced the cariogenic microbial count and dental plaque and improved the salivary pH values. AgNPs mouthwash may be used as an adjunctive measure to prevent dental caries.
1. INTRODUCTION
Dental caries is one of the most prevalent infectious diseases affecting humans. It results from the demineralization of dental hard tissues due to acids produced by cariogenic microflora in the dental biofilm, gradually leading to the localized and irreversible destruction of tooth structure [1, 2].
The microflora involved in the etiology of dental caries includes Streptococcus spp., Lactobacillus spp., and C. albicans [3]. Of these, Streptococcus mutans (S. mutans) is a major group of cariogenic bacteria that adhere strongly to the surface of the teeth through the glucans. They ferment the carbohydrates and produce acids, demineralizing the enamel and dentin [4, 5]. Lactobacillus spp. has been linked to caries development. They operate as secondary invaders with aciduric and acidogenic properties to further the progression of the carious lesion [6]. Candida albicans (C. albicans) undertake a secondary role in the progression of caries. They have a collagenolytic effect through proteolytic enzymes, particularly in dentinal caries. In addition, they are isolated from the enamel and root caries [7].
The control of dental biofilm is a pivotal approach employed in managing dental caries, primarily performed using mechanical methods. Alternative to mechanical biofilm removal, mouthwashes have been used as adjunctive antimicrobial agents in the control of dental biofilm [8].
Chlorhexidine (CHX) mouthwash has been routinely used in the prevention of dental caries and periodontal diseases [9]. CHX has antibacterial, antifungal, and antiviral properties [10]. However, numerous side effects have been attributed to CHX, including change in taste, numbness, and teeth staining. Furthermore, the prolonged use of CHX could result in resistance to oral bacteria and cross-resistance to antibiotics [11].
Silver Nanoparticles (AgNPs) are widely used in several medical fields. AgNPs possess antimicrobial properties that work against different bacteria, fungi, and viruses [12, 13]. In the dental field, AgNPs are incorporated in acrylic and composite resins [14, 15], while in the field of endodontics, gutta-percha coated with AgNPs exhibited notable antimicrobial properties [16].
Given the antimicrobial properties of AgNPs, our clinical study focused on comparing the effectiveness of AgNPs and CHX mouthwash on S. mutans, Lactobacillus spp., and C. albicans count, O’Leary PI scores, and salivary pH levels in a group of Saudi children at baseline and on the 28th day.
2. MATERIALS AND METHODS
2.1. Ethical Considerations
Ethical approval was obtained from the Institutional Review Board (IRB) of the College of Dentistry, Umm Al-Qura University (IRB No., 142-19). The study was carried out in adherence to the Declaration of Helsinki of 1975, as revised in 2000. Permission from the patients, along with their written informed consent, was obtained and signed by the parents of each child.
2.2. Sample Size Estimation
In this study, the primary endpoint was the control dental biofilm as measured by the microbial counts, PI scores, and salivary pH value. The sample size was estimated according to a parallel study conducted by Kamath et al., 2020 [17], Considering the alpha error at 5%, power at 80%, f = 0.3 (between medium and large effect sizes). The calculated sample size was 111 participants for the three groups. The sample size was estimated using G*Power Software Version 3.1.9.6., released in 2020 (Kiel University, Germany).
2.3. Study Participants
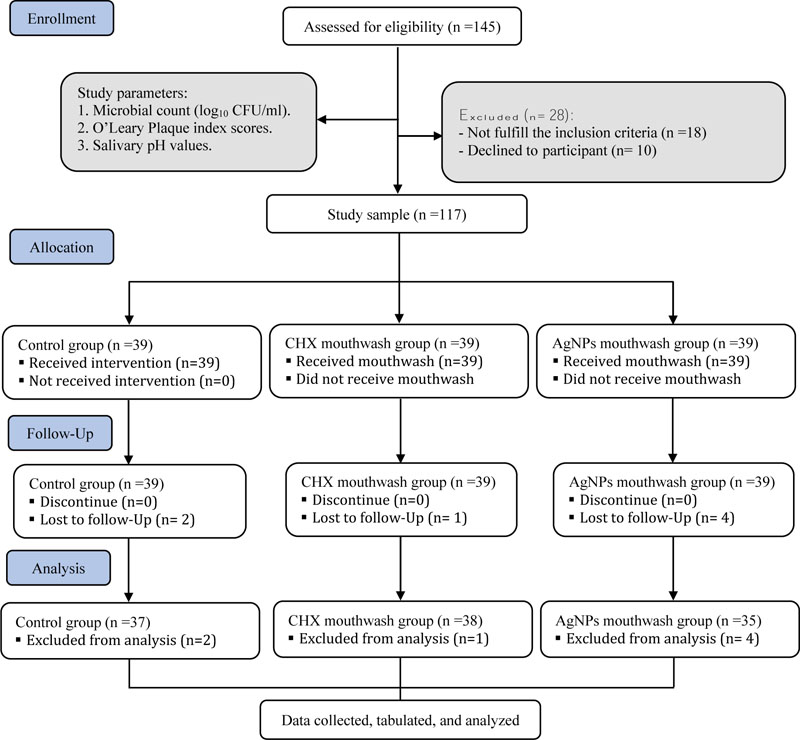
The following inclusion criteria were applied: age range of 12-15; dental caries of DMFT score ≥ 1; free of systemic disease; periodontal diseases; not receiving systemic antibiotics; corticosteroids; mouthwashes for at least 28 days before participating in the study. The study participants were recruited from a total of 145 patients attending the outpatient clinics of the College of Dentistry, Umm Al-Qura University, Makkah. Participants were randomly assigned to three groups: control, AgNPs, and CHX groups. Each group consisted of 39 participants. The CONSORT statement flow diagram was used as a guide for this study, as shown in Fig. (1).
Eligible participants underwent an oral examination; saliva samples were collected at baseline. Participants were instructed not to eat, drink (except water), or brush and floss their teeth for two hours before the sample collection.
2.4. Oral Examination
Oral examination was completed on the dental chair with a well-trained dentist, using disposable dental mirrors and explorers (Cotisen, Mould Industry Garden Empolder Section, Huanghua City, Hebei Province, China) with the chair light. The percentage of dental plaque covering the tooth surface was estimated using O'Leary PI [18]. Dental plaque and food debris that could interfere with the caries inspection were removed by a micro brush applicator (Microbrush International, 1376 Cheynne Ave. Grafton, WI 53024).
Caries status was assessed using the DMFT index prescribed by the World Health Organization (WHO) as the sum of the number of decayed teeth missed due to caries, and filled permanent teeth [19].
2.5. Stimulated Saliva Sample Collection
5 ml of whole stimulated saliva samples were collected from each participant two hours before the meal. This was carried out by asking each participant to spit for 5 minutes into sterile polypropylene tubes after lying down in a relaxing position on the dental chair. It was ensured that the saliva samples were colorless, non-contaminated with food, and free of any blood residue or tissue debris. Following this, the samples were transported to the microbiology lab in the insulated icebox at −4 °C to measure the salivary pH values and conduct the microbiological analysis.
2.6. Test Products
Nano Silver Mouth rinse comprises nanosilver, calcium, xylitol, natural flavor, and water. It has been manufactured by ElementaTM Co., 3596 Mountain Vista Parkway #3, Provo, Utah 84660, USA.
Chlorhexidine mouthwash (Avohex) contains chlorhexidine gluconate 0.2% w/v, and is manufactured by Avalon Pharma, 6294 Al Kharj Road, New Industrial City, Riyadh, KSA.
2.7. Mouthwash Administrations and Instructions
Participants in the control group were only instructed to maintain good oral hygiene by brushing regularly twice a day and abstaining from the use of any mouthwash during the study period. The instructions were delivered and explained carefully by the well-trained dentist to the participants both verbally and in a written checklist. The tooth brushing technique (Roll method) was demonstrated to each participant on a dental model afterward, and the participants’ application was evaluated before starting the study. Parents were asked to supervise them at home and review the written checklist daily. For any interruption in the instructions, the parents were informed to contact the investigators and write the event on the checklist.
Each participant in the CHX and AgNPs groups was instructed to rinse twice daily for 30 seconds with 10 ml of mouthwash [20]. They were also asked to avoid rinsing with water for at least two hours after the mouthwash, as per manufacturer recommendations.
Moreover, the participants and their parents were instructed to contact the investigators in case of any adverse reactions to the mouthwashes. A printed checklist was given to each participant to evaluate the daily consumption of the mouthwashes and the regularity of brushing. Furthermore, the participants were requested to bring back the bottles of mouthwash on the 28th day to verify task completion.
2.8. pH Characterization
The salivary pH values were estimated using a calibrated digital pH meter (Portable pH meter- HI2002-02, Hanna, Italy), equipped with an electrode and a temperature sensor (HI11310, Hanna Instruments, 84025 S. Cecilia di Eboli SA, Italy).
2.9. Microbiological Analysis
The total colony count was estimated in the whole stimulated saliva samples using serial dilution from 101 to 1010. One hundred microliters of each sample were added to 0.9 ml of saline aseptically through a sterile tube, followed by serial dilution. One hundred microliters of each dilution were spread onto Mitis Salivarius agar (MSA, Becton Dickinson Co., MD., USA) that is supplemented with sterile potassium tellurite solution 1% (Oxoid Ltd, Basingstoke, Hampshire, UK), modified de Man Rogosa and Sharpe agar (mMRS agar, Oxoid Ltd, Basingstoke, Hampshire, UK) that is supplemented with bromophenol blue (Merck), and Sabouraud dextrose agar (SDA, Oxoid Ltd, Basingstoke, Hampshire, UK) to count the S. mutans, Lactobacillus spp. and C. albicans, respectively. The MSA and mMRS plates were incubated in an anaerobe gas jar with an anaerobic gas pack (GasPak EZ, Becton, Sparks, MD) at 37ºC for 48 hours, while the SDA plates were incubated aerobically at 28ºC for 48 hours [21-23].
The total colony count was calculated and expressed as colony-forming units per milliliter (CFU/ml) of stimulated saliva, then converted to log10 CFU/ml of saliva. After the calculation, S. mutans, Lactobacillus spp., and C. albicans colonies were purified and identified using automated VITEK 2 Compact (BioMérieux, France).
During the follow-up visit on the 28th day, the microbial count, O’Leary PI, and salivary pH values were re-evaluated.
2.10. Statistical Analysis
Statistical analysis was carried out for subjects who completed the study. Descriptive statistics were presented as the mean ± standard deviation (SD) for all groups. The Kolmogorov-Smirnov test supported the normal distribution of data. The analyses of variance (ANOVA) and Tukey posthoc test were implemented to determine the changes in microbial counts, O’Leary PI scores, and salivary pH values at the baseline and on the 28th day. The p-value ≤ 0.05 denotes a significant difference between the two points. Statistical analysis was completed using the Statistical Package for Social Sciences (SPSS) software version 24.0 (SPSS Inc., Chicago, IL, USA).
3. RESULTS
As per the inclusion criteria, only 117 participants (63 males and 54 females) were eligible for the study. Two subjects in control, one in the CHX, and four in the AgNPs groups were lost to the follow-up (Fig. 1). The mean DMFT value was 3.09 ± 2.36.
At the baseline, intergroup comparisons showed non-significant differences between the study groups in terms of the microbial count, O'Leary PI, and salivary pH values (Tables 1 and 2).
On the 28th day, S. mutans, Lactobacillus spp., and C. albicans count significantly decreased from the baseline among the study groups (p-value <0.001). Moreover, pairwise comparisons revealed significant variations between the three study groups (p-value < 0.001). The CHX group showed the highest reduction in the microbial counts, followed by the AgNPs group; both intervention groups demonstrated significantly lower microbial counts than the control group (Table 1 and Fig. 2).
| Study Groups | Control (A) | CHX (B) n=38 | AgNPs (C) n=35 | p-value† | p-value‡ | ||||
| n=37 | |||||||||
| Mean ± SD | Mean ± SD | Mean ± SD | A-B | A-C | B-C | ||||
| Microbial count (log10 CFU/ml) | S. mutans count | Baseline | 7.11 ± 0.56 | 7.12 ± 0.05 | 7.13 ± 0.08 | 0.509 | 0.785 | 0.479 | 0.865 |
| 28th day | 6.25 ± 0.09 | 3.43 ± 0.15 | 4.18 ± 0.05 | < 0.001* | < 0.001* | < 0.001* | < 0.001* | ||
| Lactobacillus spp. count | Baseline | 6.19 ± 0.09 | 6.21 ± 0.15 | 6.22 ± 0.09 | 0.539 | 0.707 | 0.533 | 0.953 | |
| 28th day | 4.73 ± 0.14 | 3.61 ± 0.11 | 3.82 ± 0.14 | < 0.001* | < 0.001* | < 0.001* | < 0.001* | ||
| C. albicans count | Baseline | 2.36 ± 0.15 | 2.33 ± 0.15 | 2.32 ± 0.09 | 0.353 | 0.586 | 0.339 | 0.896 | |
| 28th day | 2.01 ± 0.29 | 0.93 ± 0.10 | 1.42 ± 0.15 | < 0.001* | < 0.001* | < 0.001* | < 0.001* | ||
| Study groups | Control (A) n=37 | CHX (B) n=38 | AgNPs (C) n=35 | p-value† | p-value‡ | |||
| Mean ± SD | Mean ± SD | Mean ± SD | A-B | A-C | B-C | |||
| O'Leary plaque index | Baseline | 45.40 ± 1.59 | 45.43 ± 2.82 | 44.42 ± 2.05 | 0.094 | 0.997 | 0.151 | 0.129 |
| 28th day | 39.76 ± 1.74 | 25.42 ± 3.53 | 34.28 ± 2.28 | < 0.001* | < 0.001* | < 0.001* | < 0.001* | |
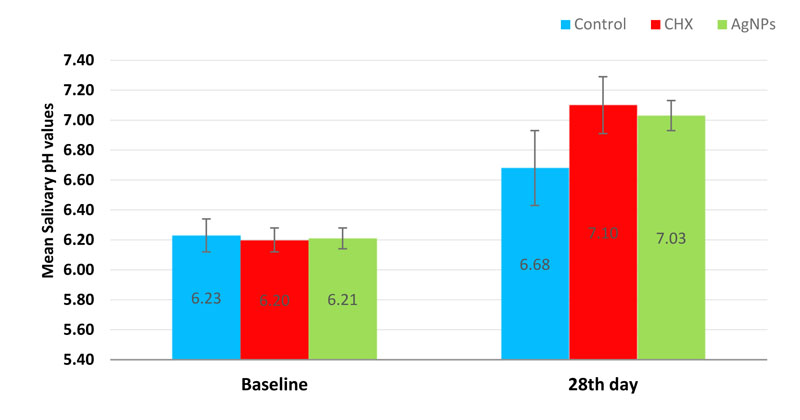
| salivary pH values | Baseline | 6.23 ± 0.11 | 6.20 ± 0.08 | 6.21 ± 0.07 | 0.548 | 0.547 | 0.703 | 0.971 |
| 28th day | 6.68 ± 0.25 | 7.10 ± 0.19 | 7.03 ± 0.10 | < 0.001* | < 0.001* | < 0.001* | 0.337 | |
In the context of O’Leary PI, intergroup comparisons on the 28th day showed a significant difference between the study groups (p-value < 0.001). CHX group had the greatest reduction in PI scores, followed by AgNPs, and finally, the control group (Table 2 and Fig. 3).
The salivary pH values recorded a significant increase on the 28th day across all the study groups. Intergroup comparisons exhibited a significant difference (p-value < 0.001) while pairwise comparisons showed a non-significant difference between AgNPs and CHX groups (p-value = 0.337) (Table 2 and Fig. 4).


4. DISCUSSION
The control of dental biofilm has a crucial role in managing dental caries. Several newer and investigational strategies for the control of dental biofilm require further research and clinical trials. These strategies include probiotics, photodynamic therapy, antimicrobial peptides, and nanoparticles [ 8, 24]. Searching the existing literature indicated the need for numerous clinical research on humans to find the most effective strategy. Hence, our study aimed to compare the effectiveness of AgNPs and CHX mouthwash in the control of dental biofilm through their effect on S. mutans, Lactobacillus spp., and C. albicans count, O’Leary PI scores, and salivary pH levels.
In our study, AgNPs and CHX mouthwash were found to have significantly reduced salivary microbial counts and O’Leary PI scores due to their antimicrobial and antiplaque effects. In addition, AgNPs and CHX mouthwash notably increased the salivary pH levels due to their abilities to reduce biofilm acidity.
Our results indicated that both AgNPs and CXH mouthwash significantly reduced S. mutans, Lactobacillus spp., and C. albicans counts on the 28th day, as compared to the control group. These results are in line with previous studies [25-27].
As per our results, CHX significantly reduces the microbial count compared to the performance of AgNPs. This finding supported the work of Elgamily et al. (2018) [28]. Similar results were obtained in vitro by mixing CHX and AgNPs with irreversible hydrocolloid impression material, wherein CHX demonstrated the highest antimicrobial activity, relative to AgNPs [29].
The antimicrobial potential of AgNPs results from the binding of the silver ions (Ag+) as a positive charge on NPs surface to the proteoglycans layer of the bacterial cell wall as a negative charge. Ag+ penetrates, perforates, denatures, and disrupts the bacterial cell wall and the cytoplasmic membrane, eventually releasing the cell organelles. Additionally, Ag+ denatures the ribosomes and inhibits protein synthesis. It also interferes with DNA replication and prevents cell multiplication [30, 31].
CHX effectively reduces the count of oral microorganisms by releasing positively charged ions that interact with the negative charges on the bacterial cell wall, thereby increasing its permeability and altering the osmotic equilibrium of the cell. This results in cytoplasmic components leaking out and, eventually, cell death [32, 33].
Our results revealed that the AgNPs and CHX mouthwash significantly reduced O’Leary PI scores on the 28th day compared to the control group. These results aligned with previous studies where AgNPs reduced dental plaque formation and adhesion by arresting the exopolysaccharide synthesis [12, 34, 35].
AgNPs penetrate the bacterial cells, impact their metabolic activity, and inhibit the production of polysaccharides, which are essential in cell-cell and cell-surface adhesion [32]. Meanwhile, CHX inhibits dental plaque formation through the bactericidal effect concomitant with its instant application as well as the bacteriostatic effect whilst it is adsorbed to the microbial plaque on the enamel surface [36, 37].
Our results showed a significant antiplaque effect with CHX, as compared to AgNPs mouthwash. This is in support of the results of Ahrari et al., (2015) [38], who reported that AgNPs-containing mouthwash was less effective against plaque forming-microorganisms, as opposed to CHX mouthwash. In contrast, Panpaliya et al., (2019) [27] concluded that AgNPs characterize a relatively higher antiplaque effect. This inconsistency may be attributed to the variation in the conditions under which each study was conducted. Note that Panpaliya et al., (2019) [27] conducted their study in-vitro, which is different from the oral cavity condition.
The results of this study revealed a significant increase in the mean salivary pH values on the 28th day in both AgNPs and CXH groups, as compared to the control group. This was in line Vazquez-Garcia et al., (2016) [39], who reported that adding AgNPs to white MTA significantly increases the pH values of deionized water medium in-vitro.
The effect of AgNPs on pH value may be attributed to their ability to reduce the biofilm acidity by releasing the calcium and phosphate ions into the plaque fluid and neutralizing the acids [40]. Likewise, 0.2% CHX mouthwash had been reported to increase the salivary pH values as it notably diminishes the acidogenicity and acidurance of the dental plaque [41].
Remarkably, our results indicated a decrease in the microbial count across the control group, in line with Relvas et al., (2014) [42]. Further, in the same context, an improvement in O’Leary PI scores and salivary pH post-twice-daily brushing for 28 days was also observed, as the participants were regularly notified about the daily toothbrushing maneuver.
Our results indicated a notable effect of AgNPs in the control of the dental biofilm in the context of reducing the microbial counts and dental plaque and improving the salivary pH values. However, further studies are needed in the future to provide stronger evidence.
Non-randomized participant assignment and relatively short follow-up time were notable limitations of our study. Furthermore, our research was restricted to S. mutans, Lactobacillus spp., and C. albicans. Consequently, further randomized control clinical trials with a long-term follow-up period comprising more cariogenic and periodontal pathogens may be of greater value to future research.
CONCLUSION
AgNPs and CHX mouthwashes effectively reduced S. mutans, Lactobacillus spp., and C. albicans counts, in addition to improving dental plaque scores and salivary pH. AgNPs mouthwash could be used as an alternative to CHX and is considered an important supplement to managing dental caries among children.
LIST OF ABBREVIATIONS
| (AgNPs) | = Silver Nanoparticles |
| WHO | = World Health Organization |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study’s ethical approval was obtained from the Institutional Review Board (IRB) of the College of Dentistry, Umm Al-Qura University, Saudi Arabia (IRB No., 142-19).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures followed were by the ethical standards of the committee responsible for human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
The parents of all 117 children provided written informed consent.
AVAILABILITY OF DATA AND MATERIALS
All data supporting the results of this study are available from the corresponding author [A.F]. on request.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


