All published articles of this journal are available on ScienceDirect.
Gelatin-hydroxyapatite Fibrous Nanocomposite for Regenerative Dentistry and bone Tissue Engineering
Abstract
Aims:
This study aimed to prepare and physicochemically evaluate as well as assess the cytotoxicity and stimulation of early osteogenic differentiation of dental pulp stem cells of gelatin-hydroxyapatite (Gel-HA) fibrous nanocomposite scaffold.
Background:
Recently, the electrospinning approach in nanotechnology has been considered due to its application in the preparation of biomimetic nanofibers for tissue engineering.
Objective:
The main objective of this study was to evaluate Gel-HA fibrous nanocomposite for regenerative dentistry and bone tissue engineering material.
Methods:
The nano-scaffold was prepared via the electrospinning method. Then, the physicochemical properties (particle size, surface charge, morphology, hydrophilicity, specific surface area, crystalline state and the characterization of functional groups) and the proliferative effects of nano-scaffolds on dental pulp stem cells were assessed. The alkaline phosphatase activity was assessed for evaluation of early osteogenic differentiation of dental pulp stem cells.
Results:
The prepared nano-scaffolds had a negative surface charge (-30 mv±1.3), mono-dispersed nano-scale diameter (98 nm±1.2), crystalline state and fibrous uniform morphology without any bead (structural defects). The nanofibrous scaffold showed increased hydrophobicity compared to gelatin nanofibers. Based on Brunauer-Emmett-Teller analysis, the specific surface area, pore volume and pore diameter of Gel-HA nanofibers decreased compared to gelatin nanofibers. The Gel-HA nano-fibers showed the proliferative effect and increased the alkaline phosphatase activity of cells significantly (P<0.05).
Conclusion:
The prepared Gel-HA nanofibers can be considered potential candidates for application in bone tissue engineering and regenerative dentistry.
Other:
Gel-HA nanofibers could be a potential material for bone regeneration and regenerative dentistry in the near future.
1. INTRODUCTION
One of the fundamental clinical problems is the regeneration of bone defects, and the final aim of studying biomaterials is the replacement of artificial organs and repairmen of damaged organs and tissues for restoration of their physiological function. Furthermore, bone defects remain challenging for dentists. Finding the perfect materials for the replacement of bone has long been the focus of bone regeneration investigation [1]. An ideal solution to the problem of clinical bone defects is the use of tissue engineering in which cells, scaffold composite materials, and biological agents are used to restore bone function and structure [2, 3].
The gold standard for bone regeneration is autografts because they provide an osteoconductive scaffold, osteogenic cells, and related growth factors, all of which are required for new bone growth. Autografts have restrictions on access and morbidity at the site of harvest. The risk of disease transmission, potential immunogenicity, and rejection are limitations of allografts and xenografts [4].
Because of the many problems related to the application of natural bone for repairing bone defects, some studies have been performed to find suitable artificial bone material. Artificial bone substitutes are produced to accurately mimic the biological properties of natural bone [5, 6]. Different kinds of synthetic bone materials have been produced for example organic polymers, metals, and glass ceramics [7-9].
Bone marrow mesenchymal stem cells (BMSCs) are utilized for bone tissue engineering due to their capability to proliferate and their high potential for osteogenic differentiation [10, 11]. Nevertheless, BMSCs are difficult to achieve and invasive. Compared to BMSCs, dental pulp stem cells (DPSCs) are easy to attain and thus shorten the healing process of trauma. Furthermore, the financial burden is much less on patients [12, 13]. DPSCs are an excellent source of MSCs for the regeneration of craniofacial bone [14].
Among different types of materials, ceramics due to brittleness and polymers sometimes due to insufficient strength, cytotoxicity, and release of undesirable products due to their destruction have not shown a completely ideal performance as a bone tissue scaffold [15].
Therefore, composites, which were mostly ceramic polymers, were introduced as the next generation of these alternatives due to their special properties such as suitable and adjustable strength and more reliable performance [16-18]. Composite scaffolds made to replace bone tissue often contain a mineral phase such as hydroxyapatite (HA) or tricalcium phosphate (TCP) along with a synthetic polymer such as polylactic acid (PLA), polyglycolic acid (PGA), poly lactic-co-glycolic acid (PLGA) or natural materials such as collagen, alginate, and gelatin [19-21].
Nanotechnology is the application of material at the atomic, molecular, and supramolecular scales that are used in various sciences such as medicine [22]. The preparation of membranes based on nanotechnology is considered a complete bone healer. Nanocomposites, in addition to possessing the properties of conventional composites, due to having a high surface area, have more mechanical interaction with the substrate and in the case of formation of chemical bonds, their number and therefore their strength will increase. From a biological point of view, experiments performed on nanocomposites have shown superior properties in these materials [23, 24]. HA could mimic the chief mineral element of the bone. A group of researchers has used the hydroxyapatite-gelatin combination as a suitable scaffold to induce ossification [25].
Various methods such as phase separation, self-assembly, and electrospinning are used to make nanostructured scaffolds for bone tissue engineering. Electrospinning has emerged as a promising and versatile method for fabricating nanofiber scaffolds for bone tissue engineering applications due to its low cost, simple operation, the production of micro-to-nano scale fibers, and processing of a wide range of polymers, which structurally mimic nanofibers of the extracellular matrix in native bone [26-28]. This method is also a capable drug delivery approach that combines the therapeutic agents in nonwoven nanofiber networks during the electrospinning process [29].
In this study, Gel-HA nano-fibers were prepared and the physicochemical properties, as well as the influence of the nano-scaffold on DPSCs viability and activity of alkaline phosphatase (ALP), were tested.
2. MATERIALS AND METHODS
All the materials utilized (gelatin, hydroxylapatite nanopowder (mean particle size of 100 nm), dimethylformamide, chloroform) were purchased from Sigma-Aldrich Co., Germany. Low-passage DPSCs were used for the biological tests, which were obtained from Shahid Beheshti University in Iran, Tehran.
2.1. Preparation Method of Nano-Fibrous Scaffold
Gelatin (8% [w/v]) was dissolved in a mixture of acetone/chloroform (15/85) using a stirrer. Then, hydroxyapatite nanoparticles were added (10% of gelatin [w/w]) and stirred for 30 minutes at 45ºC. The acquired solution was then electrospun using an electrospinning device (Nanofanavaran, Mashhad, Iran). The solution was placed in a vertical form in a capillary tube (a 5-mL syringe). For a collection of the nano-fibers over the electrospinning jets, a four-sided fixed collector was used. The electrospinning device was set as follows: 20 kV voltage, a flow rate of 1.5 mL/h, and a distance of 10 cm between a capillary tube and a collector (Fig. 1).

2.2. Characterization of Nano-scaffold
The mean size for nanofiber diameter was evaluated by the Dynamic Light Scattering (DLS) method (Malvern, United Kingdom). A small piece of the nanofibrous scaffold was freeze-dried and then 0.1 g of dried powder was weighed and dispersed in 50 mL deionized water via sonication (amplitude 20%, Power 500 W, reaction time: 20 min) at 25 °C. Experiments were performed in triplicate.
SEM images (SEM, TESCAN, Warrendale, PA) were attained to evaluate the morphologic properties of the electrospun Gel-HA nanofibers. The nanofibers were fixed to the aluminum parts with adhesive tape and coated with gold before examining the SEM images.
To evaluate the surface charge of nanofibers, zeta potential values were assessed by means of a zeta-sizer (Malvern, UK) at 25 ° C. Experiments were performed in triplicate.
The X-ray diffraction (XRD) method (Philips TW 1710 diffractometer with Cu-Kα incident radiation regulated at 40 kV and 30 mA) was used to compare the crystalline state of the material components qualitatively. The scan was performed over the 2θ range of 20°–60°, with a scanning rate of 3˚/min for all samples. The diffraction pattern was scanned over 0°–60° 2θ, at a scanning speed of 2° 2θ/min.
Moreover, Fourier-transform infrared spectroscopy (FTIR) (Thermo Nicolet-6700) was used to get the molecular structure of the nanofibers, in the range 4000–400 cm−1.
The contact angle between a water droplet and the scaffold surface was also done to assess whether nanofibers are hydrophobic or hydrophilic. A water droplet was poured onto the surface of samples (nano-fibrous scaffold and gelatin nanofibers as control) with a syringe and the whole process was recorded by a video camera. The images were taken and the contact angles were determined. Experiments were performed in triplicate.
In addition, Brunauer-Emmett-Teller (BET) analysis was applied to define the specific surface area using a BET device (JW-DA model, JWGB company- China).
2.3. Cytotoxicity of Scaffolds
Human DPSCs were attained from Shahid Beheshti University of Medical Sciences in Iran that were extracted from impacted wisdom teeth. The DPSCs were cultured in DMEM media (Gibco) with 10% fetal bovine serum (Gibco) and incubated with 5% CO2 at 37°C. For the MTT assay, the sterilized nanofibers were cut to 0.32 cm2 to match the well size in the 96-well plate and wetted with the complete culture, and then the cells (10,000 per well) were seeded onto the scaffolds in 200 μL of the medium and the plate incubated for 7 days, then all well’s contents were removed and it was added 200 μl of culture medium containing MTT (2 mg/ml PBS) and incubated for 4 hours. After, the solution was exchanged with 200 μl of DMSO. Then the absorbance of wells was read by a plate reader at 570 nm and the cell viability was considered by [A]test/[A] control ×100 [30]. The control was cells grown in the absence of nanofibers. Experiments were performed in triplicate.
2.4. Evaluation of ALP Activity
For evaluation of ALP activity, the sterilized nanofibers were cut to 9.6 cm2 to match the well size in the 6-well plate, and then the cells (25 × 104) were seeded onto the scaffolds. The activity of ALP was evaluated after 14 days in cultured DPSCs on the scaffolds. To make the lysis buffer, 1.5 M Tris-HCl and 1 mM ZnCl2 and MgCl2 6H2O were combined and diluted in 10% dH2O and 1% Triton X-100 (all materials purchased from Sigma-Aldrich, St. Louis, MO, USA). Then, the prepared lysis buffer was poured into each well and incubated at 37 °C for 30 min before being deposited overnight at 4 °C. Cell lysates were then transferred into microtubes, vortexed for 2 min, then centrifuged at 2000 rpm for 5 min. About 190 μL of p-Nitrophenyl Phosphate as alkaline phosphatase substrate (18.75 mg pNPP per 10 mL cellular solution, cat number: 4876, Sigma-Aldrich, St. Louis, Missouri, United States) was mixed with 10 μL of each cell lysate in a 96-well microplate. ALP activity absorption at 405 nm was measured using the Cytation 5TM microplate reader (BioTek, Winooski, USA). Cells grown in the absence of nanofibers were used as the control. Experiments were carried out in triplicate.
3. RESULTS
3.1. Scaffold Characterization
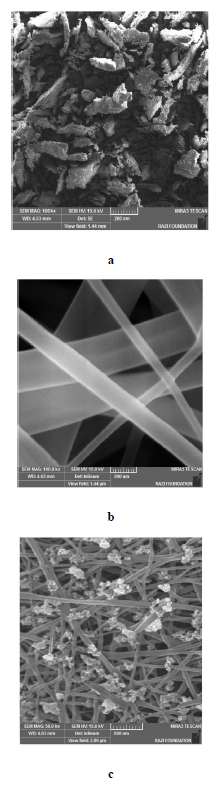
The nano-scaffolds had a mono-dispersed nano-scale diameter of 98 nm±1.2 (Fig. 2a) and a negative surface charge of -30 mv±1.3 (Fig. 2b). SEM image showed the plate-like (or sheet-like) morphology for HA nanoparticles (Fig. 3a), the uniform network-shaped fibrous morphology without any structural bead for gelatin nanofibers (Fig. 3b), and Gel-HA nanocomposite (Fig. 3c), respectively.
The prepared Gel-HA nanofibers displayed a crystalline state according to XRD peak intensities (Fig. 4a). The FTIR spectra evaluated the chemical composition of the prepared Gel-HA nanofibers, and there was no difference between the absorption bands of the bulk gelatin, HA nanoparticles, and the scaffold (Fig. 4b).


| Type of Material | Specific Surface area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|
| Gel nanofibers | 285.23 | 0.15 | 7.10 |
| Gel-HA nanofibers | 220.65 | 0.09 | 5.96 |
The results for the contact angle test showed a contact angle of 102°±0.1 for the prepared scaffold. The nanofibrous surface containing HA is then much more hydrophobic compared to the gelatin nanofibers having contact angles of 75°±0.2.
Table 1 displays the outcomes of the BET test for the prepared nanofibers. Based on the results, the specific surface area, pore volume and pore diameter of Gel-HA nanofibers decreased compared to gelatin nanofibers.
3.2. Cytotoxicity
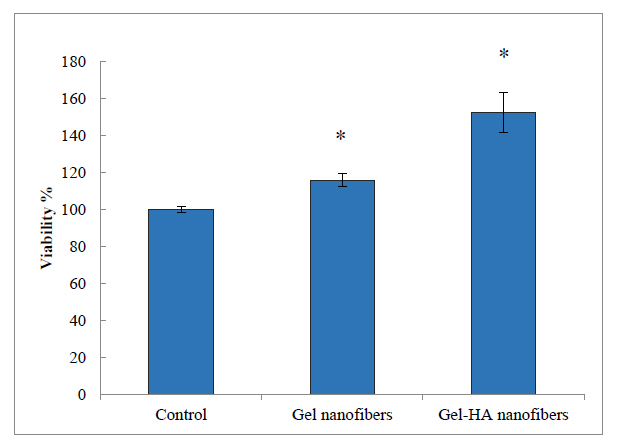
The viability of DPSCs after culturing with Gel-HA nanofibers and Gel nanofibers was tested by MTT assay (Fig. 5). After 7 days of incubation, the viability of cells increased significantly so the examined scaffolds showed the proliferative effect on DPSCs (P<0.05).
3.3. ALP Activity
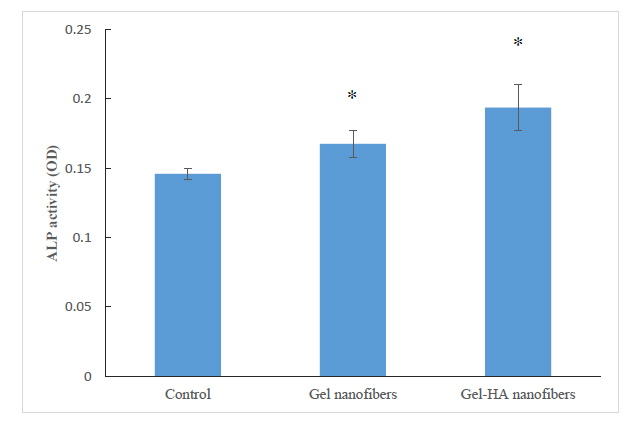
The results of the ALP activity examination on DPSCs cultured with Gel-HA nanofibers and Gel nanofibers showed a significant enhancement in the activity of ALP compared with the control (P<0.05) (Fig. 6). Furthermore, Gel-HA nanofibers showed a significant enhancement in the activity of ALP compared with Gel nanofibers (P<0.05).
4. DISCUSSION
Electrospun nanofibers, by providing a three-dimensional environment, play a role in supporting the growth of cells by mimicking the body's natural extracellular matrix (ECM). They are the best choice for biological scaffolds [30]. Electrospinning is a rapidly evolving method that demonstrates the capability to create a variety of morphologies because of its performance, simplicity, and flexibility. Processing variables for the electrospinning procedure including voltage, the distance of needle-to-collector, flow rate, as well as parameters of solution such as viscosity, the electrical conductivity of the solution, and surface tension can control the morphology of nanofibers [29]. The purpose of optimizing the electrospinning method is to identify the conditions for preparing nanofibers with a minimum diameter [31]. The results of other researchers displayed that polymeric nanoparticles, lipid nanoparticles, micelles, nanotubes, etc. can be embedded with nanofibers to improve various parameters such as drug loading efficiency, drug safety, release outline, and better fiber performance [32-35]. Nanofibers loaded with nanoparticles are similar to coaxial nanofibers because they retain the drug safe from organic solvents during the preparation and prolong the delivery period of the drug [36]. In addition, this method is simpler than coaxial electrospinning as it utilizes a nozzle. The prepared nano-fibers in this study showed a mono-dispersed nano-scale diameter of 98 nm±1.2, a negative surface charge of -30 mv±1.3, and a uniform network-shaped fibrous morphology without any structural bead. SEM image also showed the uniform spreading of HA nanoparticles on the gelatin nanofibrous matrix of the scaffold and the plate-like (or sheet-like) morphology for HA nanoparticles. According to Oliveira et al, the small size, high surface defects, poor crystallinity, and high surface energy of nuclei during nanoparticles formation lead to the agglomeration among nuclei to decrease free energy, and then the formation of sheet-like nanoparticles [37]. In a recent study, the electrospun composite scaffolds of gelatin-nanohydroxyapatite were prepared. The results showed uniform dispersion of nanohydroxyapatite on the gelatin scaffolds for 0%, 10%, 20%, and 30%; however major agglomeration of nanohydroxyapatite for scaffolds with 40% nanohydroxyapatite [38].
The surface charge of the nanoparticles has also a key part of their possessions, especially on their suspension stability. Ruphuy et al., reported that in addition to the parameters of the preparation procedure the polymer nature itself has a high influence on dispersions stability [39]. It has been reported that the negative zeta potential displays an important hopeful effect on the attachment and proliferation of osteoblasts than neutral or positive charges [40, 41]. The net charge of HA nanoparticles in an aqueous medium is due to the unequal dissolution of surface ions and may lead to either a net negative or a net positive surface charge. It is extremely dependent on the medium pH [39]. In the case of gelatin, ionization of COOH groups leads to a net negative charge of the gelatin chains [42].
As can be observed, four index peaks in the 2θ range of 26° to 50° (26.2, 32.3, 39.8 and 49.6) have appeared, which according to the standard pattern of hydroxyapatite are wider than the pure hydroxyapatite (HA) peaks (according to JCPDS NO: 09-432), indicating the low crystallinity of hydroxyapatite in the prepared composites [43]. This can be due to the amorphous nature of the gelatin and also because of the nanometer size of the scaffold [44]. Hezma et al. reported comparable results for polylactic acid–hydroxy apatite–curcumin nanocomposites [45].
The FTIR peaks for gelatin at 2900 cm-1corresponds to the tensile vibration of the H-C, the peak at 1640 cm-1 is related to the tensile vibration of the O=C bond of the amide group, and the peaks at 1490 cm-1and 1520 cm-1 show the tensile vibration of the H-N bond of the amide group. Phosphate groups show intensive absorption bands at 560 and 600 cm-1 and 1000–1100 cm-1. Peaks at 1413.82 cm−1 and 1460 cm−1are due to carbonate groups in the HA [46]. A wide absorption band in the range of 3439 cm-1 is attributed to the presence of water molecules [43]. The FTIR spectra of hydroxyapatite and gelatin are absorbed in the prepared scaffold. Sharifi et al. reported similar results for hydroxyapatite–gelatin/curcumin nanofibrous composites [47].
The nanofibrous surface containing HA (contact angle of 102°) showed more hydrophobicity compared to the gelatin nanofibers (contact angles of 75°).
According to reports, electrospinning of nanoparticles into nanofibers leads to an increase in the hydrophobicity of the surface, which is of excessive attention for uses such as biomedicine. Enhanced hydrophobicity rises from the inherent roughness of nanofibers formed by their porous structure and small diameter [48].
Specific surface area, total volume, and size of the pores for nanofibers show significant influence on cell adhesion, growth, and proliferation. Therefore, any incorporation of active substances influenced these processes. There are different methods to determine the specific surface area for nanofibers including imaging methods, optical microscopy, electron microscopy, mercury porosimetry and Brunauer, Emmett and Teller (BET) analysis. All these methods are functioning for the determination of specific surface area for nanofibers; however, the BET method can be powerfully used for specific surface area determination of nanofibers with pores diameter of 1.6–10 nm [49]. Li and He prepared ZrO2 nanofibers by electrospinning method and characterize the specific surface area and total pore volume of nanofibers by the BET method. According to their results, the prepared nanofibers showed a large specific surface area of 268.423 cm2/g with a total pore volume of 0.146 cm3/g [50]. Also, the reason for the reduction of the pore size may be due to the presence of HA in pores of Gel-HA nanofibers compared to gelatin nanofibers [51].
Kim and his colleagues have done extensive studies on gelatin and hydroxyapatite. In one of their studies, they showed that hydroxyapatite and gelatin composites showed good morphological and mechanical properties and cellular responses in vitro in terms of hard tissue regeneration [52]. In another study from this group, it was found that in vitro, osteoblastic MG63 cells attached to Gel-HA nanocomposites proliferate significantly more than their normal form. The activity of ALP and osteocalcin produced by cells in nanocomposite scaffolds was significantly higher than in conventional composite scaffolds [53].
Our results displayed that the Gelatin-hydroxyapatite fibrous nanocomposite fibers enhanced the early osteogenic differentiation of cells. Increased activity ALP shows that this membrane may increase the osteogenic differentiation of DPSCs on Gelatin-hydroxyapatite fibrous nanocomposite fibers.
Azami et al. studied nanostructured scaffolds using hydroxyapatite and gelatin that has a 3D homogeneous interconnected porous structure with 82% porosity and pore size of 300 to 500 μm. It has also been revealed that mechanical indicators are within the range of spongy bones [54].
Chen et al. produced core-shell nanofibers of hydroxyapatite/gelatin-chitosan to mimic both the microenvironment and the chemical composition of natural bone and suggested it as a promising material for the promotion of osteoblast growth in tissue engineering of bone [55].
In the present study, the prepared nano-scaffolds did not have a toxic effect, which had a proliferative effect on DPSCs as well as stimulated early osteogenic differentiation of DPSCs. Thus, Gel-HA nanofibers can be a capable nano-scaffold for bone regeneration. A combination of different materials to decrease the fiber degradation rate is likely to help increase tissue regeneration. Nevertheless, more in vivo investigations are essential.
Salifuet al. examined the human fetal osteoblast cells on gelatin-hydroxyapatite electrospun fiber scaffolds at different hydroxyapatite concentrations of 0, 10, 20, and 25 wt %. Their results showed that 25 wt % hydroxyapatite-gelatin scaffolds led to the greatest cell attachment, proliferation of cells, and production of ECM [56].
CONCLUSION AND FUTURE OUTLOOKS
Nanotechnology in dentistry is doing its best to apply new signs of advances in dental practice and tissue engineering. With the increase of advanced research and a deeper understanding of electrospinning set-ups, it is likely that future “smart bone healing devices” capable of treating all features of bone defects for actual clinical use could be achieved. This study demonstrated that gelatin-hydroxyapatite fibrous nanocomposite could be a potential approach for bone regeneration and regenerative dentistry in the near future.
LIST OF ABBREVIATIONS
| Gel-HA | = Gelatin-Hydroxyapatite |
| DPSCs | = Dental Pulp Stem Cells |
| HA | = Hydroxyapatite |
| TCP | = Tricalcium Phosphate |
| PLA | = Polylactic Acid |
| PGA | = Polyglycolic Acid |
| PLGA | = Poly Lactic-coglycolic Acid |
LIMITATION OF STUDY
The current study specifies the in vitro potential of mineralized gelatin as a hard tissue implant. The study can be improved by changing the nanofiber composition. Animal and human studies are needed for verification of the actual therapeutic use of this new substance.
AUTHORS' CONTRIBUTIONS
The study was planned by SMD and SSH carried out data collection. SS, EDA, and RK have been involved in the research steps or the drafting and revision of the manuscript. SMD and EDA are the corresponding authors. All authors read and approved the final manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The Ethics Committee of the Tabriz University of Medical Sciences. (IR.TBZMED.VCR.REC.1401.028) approved this study.
HUMAN AND ANIMAL RIGHTS
No animals/human were used that are the basis of this study.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the finding of this study are available within the article.
FUNDING
None.
CONFLICT OF INTEREST
Solmaz Maleki Dizaj and Simin Sharifi are on the Editorial Advisory Board of the The Open Dentistry Journal.
ACKNOWLEDGMENTS
The data of the present study was derived from a thesis (number 69816), which was supported by the Vice-Chancellor for Research at Tabriz University of Medical Sciences and the authors would like to appreciate their support.


