A New Natural Antimycotic Agent is Effective Against Oropharyngeal Candidiasis: The VIPROCAN Study
Abstract
Background:
The incidence of community and nosocomial candidiasis has dramatically increased in the last two decades. There are multiple treatments for this infection, but the toxicity of some and the induction of resistant strains require the development of new compounds.
Objectives:
With the aim of reducing the Candida population in the oropharyngeal cavity, we have formulated a toothpaste with VG-01 agent, composed of a mixture of carnosic acid (CA) and propolis (PP).
Methods:
We investigated the ability of VG-01 toothpaste to minimize and stabilize fungal presence in 21 patients diagnosed with clinical oropharyngeal candidiasis.
Results:
Our data indicate that VG-01 toothpaste showed an effect not only against the most frequent species of Candida, C. albicans, but also in the other species analyzed. 82% of patients stated that they would continue using it outside the study.
Conclusion:
Our data demonstrate that VG-01, composed of CA and PP is a potential antimycotic agent effective against the most common species that cause oropharyngeal candidiasis present in clinical practice.
1. INTRODUCTION
More than 1.7 billion people are affected by the most common fungal infections, which cause damage to the skin and nails [1]. The deadliest clinical mycology diseases are caused by Cryptococcus (600,000 per year), Pneumocystis, Candida, and some Aspergillus [2]. Fungal infections are classified as either primary pathogens, which develop in immunocompetent hosts, or opportunistic infections, which develop when the immune system is compromised [3]. Although fungi have been considered secondary opportunistic pathogens, the incidence of community and nosocomial mycosis has dramatically increased in the last two decades [4]. The extension of the immunocompromised population (including AIDS patients, neonates, or individuals suffering debilitating diseases or those subjected to invasive surgery and prolonged hospitalisation), the frequent isolation of non-pathogenic fungal species as the cause of clinical outbreaks, and the limited arsenal of antifungal compounds available, together with the growing emergence of resistant strains are, among others, contributory factors that explain this worrying scenario [5].
The genus Candida, particularly Candida albicans, remains the most prevalent etiological agent of systemic mycosis in humans. Several Candida spp. are considered commensal members of the human microbiota, but they can cause mucocutaneous candidiasis, involving skin and mucosal layers [6]. Apart from the external surfaces, Candida albicans is a normal commensal of the upper respiratory, gastrointestinal, and genitourinary tracts; however, under certain circumstances, it could convert into a human pathogen. Both mucocutaneous and disseminated candidiasis have serious implications for the prognosis and treatment in clinical practice [7].
Three main families of antifungals are customarily used in medical chemotherapy: azoles (fluconazol or ketoconazol), polyenes (Amphotericin B or nystatin), and echinocandins (caspofungin or micafungin) [8]. However, their practical application gives rise to important problems. As a result of the structural and metabolic similarities between fungus and host cells, antifungal targets are limited, and selective toxicity is reduced, resulting in several compounds causing side effects and harm in patients [9]. Furthermore, some dangerous fungi are refractory to certain compounds (i.e., Cryptococcus sp and echinocandins), and the above-mentioned isolation of drug-resistant strains complicates the therapy, like the well-described resistance to azoles shown by Candida strains in patients with advanced HIV [10]. It is also worth noting that the estimated cost of septicemic mycosis ranges between ₤30,000 and ₤50,000 per patient [11].
For these reasons, a search for new, more potent, and selective antifungal compounds is an urgent need for antimycotic therapy [12]. In this context, plants have traditionally been used as a source of natural products endowed with antimicrobial activity and successfully applied for health care [13]. Our group has just carried out an in-depth study regarding the synergistic fungicidal action displayed by an elaborated mixture of carnosic acid (CA) and propolis (PP) (here termed as VG-01) [14]. Both natural agents have antifungal and antibacterial properties [15], as well as antioxidant properties, and are widely used as food additives and prodrugs due to their beneficial effects on human metabolism and lack of off-target toxicity [16, 17]. Our previous study was restricted to in vitro models; for this reason, we now evaluate the clinical efficacy of VG-01 integrated into standard toothpaste to reduce the population of Candida albicans in patients with oropharyngeal infection. Our data of the VIPROCAN study reveal that VG-01 added in the toothpaste could be applied during daily oral patient hygiene, reducing oropharyngeal fungal presence.
2. MATERIALS AND METHODS
2.1. Study Design
We designed a longitudinal, unicentric, open, single-arm study to compare the levels of Candida albicans in saliva before (day 1), after the use for 24 h (day 2), and after 14 days (day 15) of the CA treatment in combination with the bioactive components of propolis, both incorporated in the above-mentioned vehicle. Fungal presence was confirmed by PCR (Fig. 1).
Inclusion criteria were ages between 18 and 80 years old, with clinically suspected candidiasis and with at least 90% of the original teeth; a general condition measured by the Karnofsky scale greater than or equal to 80%; steroids treatment, or xerostomia, or type 2 diabetes mellitus or frequent use of broad-spectrum antibiotics, being treated with chemotherapy or drugs that weaken the immune system and ability and willingness to comply with the visits planned within the study as well as the plan for the use of the toothpaste and all the procedures of the study. Major exclusion criteria were patients diagnosed with HIV or any other infectious-contagious pathology requiring acute treatment, concurrent use of an antifungal agent, active infection other than oropharyngeal candidiasis in the oral cavity at the moment of starting the study or in the previous 14 days, current or previous history of head and neck cancer regardless of whether they have received adequate treatment, any chronic medical or psychiatric condition or laboratory abnormality that may increase the risk associated with participation in this study or that, in the investigator's judgment, may interfere with the interpretation of the results of this study. These included but were not limited to chronic active hepatitis, uncontrolled hypertension, unstable angina, concurrent neurodegenerative disease, acute myocardial infarction or congestive heart failure within the past 3 months, uncontrolled arrhythmias, dementia, or altered mental status that makes it difficult to understand the meaning of informed consent or compliance with the requirements of the protocol, evidence of alcohol or drug abuse, participation in any other study with any drug or nutritional supplement within 60 days prior to enrollment in this study and hypersensitivity or allergy to PP or CA or any of the components of the toothpaste supplied.
The study was done in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. All patients provided written informed consent. Approvals for the study protocol (and any modifications thereof) were obtained from the independent ethics committee (Ethic code: 16.06.0961).
2.2. Clinical Procedures
Participants that satisfied all inclusion and exclusion criteria (N=23) received the VG-01 toothpaste, together with a toothbrush, to be tested at home. Patients would brush their teeth and tongue with 0.5 g of VG-01 toothpaste for 2 minutes, three times daily for 2 weeks. The use of additional oral care products was forbidden during the study. Three visits to the Hospital were established for each patient, and all study restrictions were checked before taking the biological samples. The salive was obtained after a 15 seconds mouthwash, using 15 cc of autoclaved miliQ water in a 50 ml Falcon sterile tube. In addition, representative photographs of the oral area with suspected candidiasis were taken. Finally, at visit 3, patients were asked about their level of satisfaction with the study.
2.3. Sample Recollection and DNA Extraction
Saliva samples were centrifuged at 20,000 g for 5 minutes, and the supernatants were removed. Samples were then snap-frozen and stored at -80ºC until DNA extraction was required. DNA was isolated by Yeastar Genomic DNA (Zymo Research) following manufacturer’s recommendations.
2.4. Fungal Presence Analysis
In order to be able to establish a correlation between the DNA amplification values and CFUs, a sample of standard C. albicans was thawed and seeded on Agar with the corresponding medium for 24 hours. Then a colony was collected and spread in liquid medium O/N. Afterward, paired serial dilutions were performed; one of them was used to isolate total DNA, and the other was seeded on Agar. After 24 hours, a colony count was performed on all cultures on Agar.
After total DNA isolation and quantification by the Nanodrop 2000 (Thermo Scientific), the specific area of ITS2 was amplified by PCR with a pair of general fungal primers on ITS2: the forward primer ITS3 (5’-GCATCGATGAAGAA CGCAGC-3’) and reverse primer ITS4 (5’-TCCTCCGCT TATTGATATGC-3’) [18] concurrent with a C. albicans specific Taqman probe (5’-FAM-ATTGCTTGCGGCGGTAA CGTCC-TAMRA-3’) [19], with the following cycling conditions: 50 °C for 2 min, 95 °C for 10 min and 40 cycles of 95 °C for 15 s and 60 °C for 1min in the 7900HT Fast Real-Time PCR System (Applied Biosystem).
To calculate the relative values between two samples, a standard curve was made with DNA previously extracted from a C. albicans population of known concentration.
2.5. VG-01 Incorporated into Toothpaste and Stability
The toothpaste was elaborated according to usual methods and using habitual components of this type of product: firstly, the hydrosoluble and liposoluble components are mixed in separate stirred tank reactors. Afterward, both phases are put together with continuous stir until a homogenous mixture is reached. Finally, the active compounds are added.
In this case, the main ingredients were water, glycerin, flavouring, thickener, aromatizing agent, lightening, natural stabilizer, and VG-01 dissolved properly.
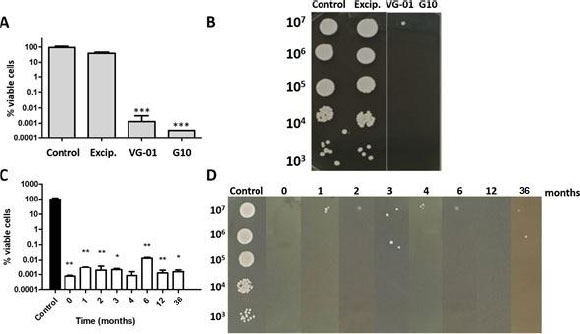
Subsequently, VG-01 toothpaste was formulated with VG-01 (1% CA + 2% PP) as the only active ingredient and was tested in viability assays. VG-01 toothpaste stability was monitored for 3 years, controlling its rheological and organoleptic properties, pH, and fungicidal action. In particular, the last parameter was performed by determination of cell viability percentage. An additional toothpaste named G10 was also tested, containing a double concentration of VG-01 (2% CA + 4% PP). For this purpose, cultures were grown at 37ºC in Yeast Peptone Dextrose (YPD) until they reached exponential phase (OD 600 nm= 0.8–1.0), then divided into several identical aliquots, and treated with VG-01 or G10 toothpaste for 1h. Cell viability was determined in samples diluted appropriately with sterile water by plating in triplicate on solid YPD after incubation for 1-2 days at 37ºC. Between 30 and 300 colonies were counted per plate. Survival percentages were normalized to control samples (100% viability). Colony growth in the solid medium was tested by spotting 5 μl from the respective ten-fold dilutions onto YPD agar. Then, the plates were incubated at 30ºC and scored after 24 h.
As it can be observed in Fig. (2), the VG-01 toothpaste activity and stability were outstanding. It is important to highlight that the toothpaste was stored under environmental conditions.
2.6. Statistical Methods
Clinical outcomes were measured by statistical analysis using the first visit as the control. The study was performed using a 2-tailed Student t-test. Furthermore, the data are presented as means ± standard deviation. Statistical significance was considered when the p-value provided by the different statistical tests was below the arbitrary value of 0.05.
3. RESULTS
3.1. VG-01 Antifungal Activity and Stability are Maintained Over the First three Years
To determine the VG-01 and G10 toothpastes’ antifungal potential, we first discarded the possible effects of the toothpaste excipients. As seen in Fig. (2A and B), the percentage of viable cells only decreased when the active compounds were added (VG-01 and G10). Once we checked that, we monitored VG-01 activity and stability upon 3 years, showing the same degree of C. albicans cell death (Fig. 2C and D).
3.2. VG-01 is Effective Against C. albicans and other Species of the Genus Candida
To further evaluate the VG-01 toothpaste effect, we designed a pilot clinical study in which 23 patients joined, and 21 were available for efficacy analysis. Our results pointed out that the treatment with VG-01 toothpaste improved clinical oropharyngeal lesions observations in all the patients studied from the first application (visit 2) and are maintained over time (visit 3) (Fig. 3). As is shown in Table 1, C.albicans was detected by the specific probe used in PCR in 66.66% of the patients prior to VG-01 treatment. For example, other Candida species, Candida glabrata, have been amplified by our primers in five saliva samples of those patients [18].
| Candida albicans | Other Candida species | Clinical outcome | ||||||||||||
| Patient ID | Sex | Age | Comorbidities | Oral hygiene | Other treatment | Immune suppression | Visit 1 | Visit 2 | Visit 3 | Visit 1 | Visit 2 | Visit 3 | Visit 2 | Visit 3 |
| Patient 1 | M | 61 | No | TID | No | No | Yes | No | No | No | Yes | No | B | B |
| Patient 2 | F | 71 | Xerostomia | Twice a day | No | No | Yes | Yes | Yes | No | Yes | No | B | B |
| Patient 3 | F | 87 | Xerostomia | TID | Xeristar, atarax, eutirox, spacmoctyl | No | Yes | Yes | Yes | No | Yes | No | = | W |
| Patient 4 | M | 62 | Xerostomia Inhalers |
TID | Chemotherapy | Yes | Yes | Yes | Yes | Yes | Yes | Yes | B | B |
| Patient 5 | F | 84 | Xerostomia | TID | Chemotherapy | Yes | Yes | Yes | Yes | No | No | No | B | B |
| Patient 6 | F | 68 | Xerostomia | Twice a day | Chemotherapy | Yes | Yes | Yes | Yes | Yes | No | No | B | B |
| Patient 7 | M | 55 | Xerostomia Bone marrow transplant |
TID | Cyclosporine | Yes | No | No | Yes | Yes | Yes | No | B | B |
| Patient 8 | M | 80 | Type 2 diabetes | Twice a day | No | No | Yes | Yes | No | Yes | No | Yes | W | B |
| Patient 9 | F | 45 | No | TID | No | No | No | No | No | Yes | Yes | Yes | B | W |
| Patient 10 | F | 81 | Type 2 diabetes Inhalers Xerostomia |
TID | Septrin Forte | No | No | No | No | Yes | Yes | Yes | W | B |
| Patient 11 | M | 72 | No | Twice a day | High spectrum antibiotics | No | Yes | Yes | Yes | Yes | Yes | Yes | W | = |
| Patient 12 | F | 70 | Inhalers Xerostomia |
Twice a day | Steroids | No | NS | NS | NS | NS | NS | NS | - | - |
| Patient 13 | M | 63 | Inhalers | Twice a day | No | No | Yes | No | Yes | No | Yes | No | B | B |
| Patient 14 | M | 84 | Inhalers | Once a day | High spectrum antibiotics | No | No | Yes | No | Yes | Yes | Yes | W | B |
| Patient 15 | M | 48 | Xerostomia | TID | Chemotherapy Radiotherapy |
Yes | No | No | No | Yes | Yes | Yes | W | W |
| Patient 16 | M | 79 | Type 2 diabetes Inhalers Xerostomia |
TID | High spectrum antibiotics | No | NS | NS | NS | NS | NS | NS | - | - |
| Patient 17 | F | 57 | Xerostomia | Twice a day | No | No | No | No | No | Yes | Yes | Yes | B | B |
| Patient 18 | M | 80 | Inhalers Xerostomia |
TID | No | No | Yes | Yes | Yes | Yes | No | Yes | B | B |
| Patient 19 | M | 48 | No | TID | No | No | Yes | Yes | No | No | Yes | Yes | B | B |
| Patient 20 | M | 57 | Xerostomia | TID | Chemotherapy | Yes | No | No | No | Yes | Yes | Yes | B | B |
| Patient 21 | F | 63 | Xerostomia | TID | Radiotherapy | Yes | Yes | Yes | Yes | No | No | No | W | W |
| Patient 22 | F | 62 | Xerostomia | Twice a day | Chemotherapy | Yes | Yes | Yes | Yes | No | No | No | B | B |
| Patient 23 | F | 56 | Inhalers Xerostomia |
Twice a day | High spectrum antibiotics | No | Yes | Yes | Yes | No | No | Yes | B | B |
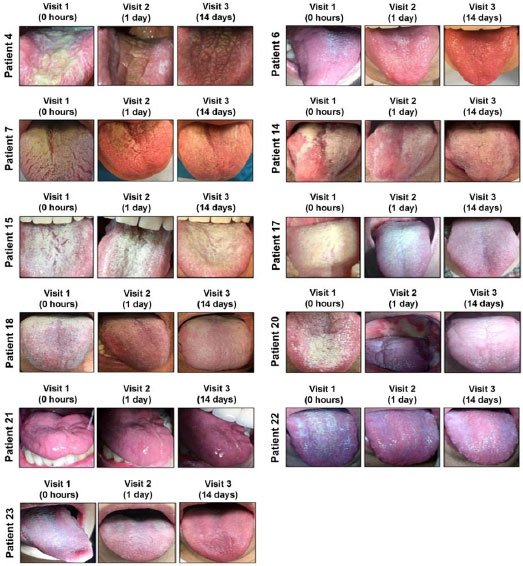
Although only in one patient (Patient 1) the toothpaste has completely eradicated fungal infection, most subjects (76.3%) finished the VG-01 treatment with better clinical outcomes.
Our data indicate that VG-01 toothpaste showed an effect not only against the most frequent species of Candida, C. albicans, but also in the other species analyzed (Fig. 3).
3.3. Most of the Patients will use the Toothpaste out of the Trial
In order to improve existing VG-01 toothpaste features, we developed a patient satisfaction survey, in which we have included sensory analyses. The rheological and organoleptic properties were evaluated by sensory analyses in which we selected some parameters to identify the visual, gustative characteristics, and acceptability of the product.
| Questions About VG-01 Toothpaste | Very Satisfied | Satisfied | Not Satisfied | Neither Satisfied nor Dissatisfied |
| How satisfied are you with the flavour? | 12.5% | 25% | 56.25% | 6.25% |
| How satisfied are you with the color? | 18.75% | 6.25% | 50% | 25% |
| How satisfied are you with the texture? | 31.25% | 25% | 31.25% | 12.5% |
| How satisfied are you with the amount of foam? | 25% | 0% | 75% | 0% |
| Yes | No | N/A | ||
| I believe that I will use it out of the study | 81.25% | 12.5% | 6.25% | |
| Symptoms have improved at 1st visit | 71% | 28% | ||
| Symptoms have improved at 2nd visit | 57% | 43% | ||
| Flavour | N/A | |||
| Recommendations to increase its use out of the study | 50% | 50% |
Although most of the patients were not satisfied with the flavour (56.25%), with the amount of foam (75%), or with the color (50%), neither of these characteristics are considered quality indicators. It is noteworthy to mention that 15 of the 21 patients have improved symptoms at 1st and 57% at 2nd visit. 81.25% of the patients enrolled in the present study would continue using it out of the study, which reveals their high degree of satisfaction (Table 2).
4. DISCUSSION
Candida is present in the oral microbiome of more than 50% of healthy people [20]. However, several factors could cause a pathogenic overgrowth [21].
There is a state of balance in the oral cavity of the microbial population. However, opportunistic microorganisms often grow and initiate a disease [22]. Although several types of oral candidiasis exist, the most common is pseudomembranous candidiasis, also known as thrush [23]. The main feature of this kind of oral infection is the formation of white plaques in the oropharyngeal space, which treatment starts with good oral hygiene and topical antifungal compounds [24].
In the present study, we demonstrate that the addition of the compound VG-01 in a base toothpaste reduced and/or controlled thrush in the population studied. The main improvement was evidenced during the first 24 h of treatment when the majority of patients presented clinical response, reducing the colonization of Candida species in oral cavities.
It is important to note that we have corroborated the effectiveness shown in our previous report in vitro [14] and that saliva does not affect antifungal properties and activity.
As with other similar studies, it is not possible to confirm continued use of the intervention product, so it is possible that the non-effectiveness shown in four of the patients is simply a consequence of patients not following the treatment consistently once they have observed some improvement.
On the other hand, the study's design allowed for the recruitment of patients with diagnoses of several Candida species, with infection by the Albicans species being the most frequent [25]. In this sense, the treatment had similar efficacy in all the Candida species that were isolated.
The number of patients who finally agreed to participate in this sub-study was small, limiting any conclusion but confirming that clinical observation and the molecular method could reliably diagnose the presence or absence of fungal infection. Our efficacy data are similar to those published in a few studies using only clinical observation as an experimental efficacy parameter [26]. Moreover, antifungal efficacy depends on the oral lesion and formulation [27].
Recent reports are promoting the use of oral health outcome measures, and patient survey satisfaction has gained attention due to the improvement in clinical practice [28]; for this reason, we have performed a survey concerning aspects about toothpaste use at the end of the study. Although the patient questionnaire highlights the need to improve the taste, texture, and amount of foam, the results obtained are consistent with the molecular results. Most patients found an improvement after 24 h and/or 15 days of treatment (71% and 57%, respectively). Notably, 82% said they would use it outside the study, showing high satisfaction with the VG-01 toothpaste treatment.
CONCLUSION
Our data demonstrate that VG-01 toothpaste composed of 1% of CA and 2% of PP has had antimycotic activity since the first application in most of the patients. Patients reported a benefit against the most common species that cause oropharyngeal candidiasis present in clinical practice. The use of this agent in the toothpaste was well tolerated and could be applied during daily oral patient hygiene. In terms of value-based health care (VBOHC), the anticandidal activity of VG-01 toothpaste would reduce the costs involved to public health and private health insurance.
Data of the present pilot study could provide invaluable information for dental care to expedite it and may provide the basis for new antifungal infections treatment.
AUTHORS' CONTRIBUTION
Conception and design: Cristobal Belda-Iniesta and Angel Ayuso-Sacido(II) Administrative support: Alejandra Argüelles(III) Provision of study materials or patients: Alejandro Tovar Lozada, María Lara Martínez-Gimeno and Mercedes Arnás-Rodríguez(IV) Collection and assembly of data: Josefa Carrión Navarro(V) Data analysis and interpretation: Josefa Carrión Navarro and Noemí García-Romero(VI) Manuscript writing: All authors(VII) Final approval of manuscript: All authors
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Research Ethics Committee of HM-Hospitales, Spain (Ethic code: 16.06.0961-GHM).
HUMAN AND ANIMAL RIGHTS
The study was done in accordance with Good Clinical Practice guidelines. No Animals were used in this research. All human research procedures were followed in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
All of the participants signed a free and informed consent agreement before taking part in the study.
STANDARDS OF REPORTING
STROBE guidelines and methodologies were followed in this study.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
The study is funded by Vitalgaia S.L., Spain.
CONFLICT OF INTEREST
AA is an employee of Vitalgaia España S.L. The rest of the authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
This work was supported by Vitalgaia España, SL. VG-01 is subjected to patents: EP 3272344. “Synergistic composition comprising propolis and carnosic acid for use in the prevention and treatment of candidiasis”. 31/01/2019. US 10,272,120 B2. “Synergistic composition comprising propolis and carnosic acid for use in the prevention and treatment of candidiasis”. 30/04/2019. CA 2,975,047. “Synergistic composition comprising propolis and carnosic acid for use in the prevention and treatment of candidiasis”. 25/06/2019.


