All published articles of this journal are available on ScienceDirect.
Evaluation of the Effect of Cold Plasma Treatment on the Microshear Bond Strength of Composite Resin Restorations to Dentin using Different Adhesive Systems and the Effect of Thermocycling
Abstract
Introduction:
Currently, non-thermal plasma is used to modify the enamel and dentin surfaces to improve the bonding surface to dental composite resins. Non-thermal plasma creates a hydrophilic surface, decreases the contact angle, and improves the bonding quality. The present study aimed to evaluate the microshear bond strength (µSBS) of composite resins to dentin using different adhesive systems.
Materials and Methods:
Bovine incisor teeth were randomly assigned to three groups of G-Premio, Clearfil SE Bond, and Adper Single Bond adhesive groups after preparation. Each group was divided into two subgroups in terms of argon plasma surface preparation, and each subgroup was divided into two groups in terms of thermocycling (n=12). The microshear bond strength of the samples was determined using a universal testing machine. Three-way ANOVA was used to analyze the effect of the adhesive, plasma preparation, and thermocycling. Post hoc Tukey tests were used for two-by-two comparisons of µSBS. Statistical significance was set at P <0.05.
Results:
The results of the µSBS test showed that the application of plasma resulted in a significant increase in the mean µSBS in the G-Premio group, with no significant increase in the Clearfil SE bond and Adper Single groups. The effect of thermocycling after plasma application was significant only in the Adper Single group.
Conclusion:
The application of plasma might increase the bond strength of composite resins to dentin. However, further studies are necessary.
1. INTRODUCTION
More than 260 million direct composite resin restorative procedures are carried out worldwide annually. These restorations are the first choice for direct anterior and posterior restorative procedures due to their satisfactory esthetic appearance, preservation of the tooth structure compared to indirect restorations, repair capacity, and ease of application [1]. The durability of the bond between the resin and tooth structure is necessary for the clinical longevity of adhesive restorations. However, the long-term stability of the dentin attached to resin is questionable. There is a consensus that resin-dentin bonds achieved by hydrophilic dentin adhesives disintegrate over time [2].
Microleakage is the main reason for increased tooth sensitivity and the progression of recurrent caries beneath restorative materials. Microleakage might occur due to the gap between the tooth structure and restorative materials, dentinal fluid, the properties of restorative materials, including dissolution, thermal expansion coefficient, polymerization shrinkage, and the cavity design and the technique used to apply the restorative materials. Microleakage might lead to pulpal inflammation in vital teeth due to bacterial toxins. Besides, the longevity of the restoration decreases due to bacterial colonization at tooth-restorative material gaps or within the dentinal tubules [3].
If a higher bond strength to dentin is necessary, modifications should be made in the currently used dentin bonding techniques. One of the reasons for the introduction of different generations of bonding agents with varying properties and different preparation techniques of dentin surface preparation, including the application of lasers with different wavelengths and times, different denting etching agents, or the application of non-thermal plasma, is to improve the bond strength longevity [4, 5].
Recently, the use of cold plasma spray (CPS) has been suggested for surface preparation. This technique improves the surface characteristics of dental materials and different substrates and increases their wettability. Surface preparation with CPS increases the surface energy, which is higher than other mechanical and chemical methods, preparing a clean and efficacious surface for bonding, with no change in the general inherent properties of the material [6].
Plasma is the fourth state of matter [7]. It is categorized into thermal and non-thermal categories regarding the relative temperature of electrons, ions, and neutral particles. In the thermal plasma, the electrons and heavy particles have the same temperature, i.e., they are in thermal equilibrium. On the other hand, in non-thermal plasma, the ions and neutral particles are at very low temperatures [sometimes at room temperature], while they have very hot electrons. In recent years, cold atmospheric plasma (CAP) sources have been introduced, which have made it possible to develop plasma treatment on living tissues [8].
The current use of plasma in dentistry relies on the two techniques of pretreatment and direct application. A study showed that the plasma treatment of the dentin surface increased the bond strength; however, plasma application for >100 seconds decreased the bond strength [9].
A study evaluated the effect of plasma treatment on dentin and improving the bonding surface for self-etching adhesives and showed that after applying plasma, the dentinal tubules’ orifices were wider, and the hybrid layer was thicker, with the formation of larger resin tags, indicating improvements on the bonding surface between the dentin and adhesive [10].
Besides, plasma results in polymerization. The synthesized polymers have exhibited more cross-linking and a high polymerization rate after being exposed to plasma [11]. More recently, non-thermal plasma brushing in the polymerization of self-etch adhesives has been reported to be effective, with no adverse effects of water on the degree of conversion in plasma-treated samples [12].
The present study aimed to determine the microshear bond strength of composite resin restorations to dentin using different adhesive systems and evaluate the effect of plasma application and thermocycling on µSBS.
The null hypothesis of the study stated that plasma application and thermocycling do not affect the microshear bond strength of composite resins to dentin.
2. MATERIALS AND METHODS
The present in vitro study was undertaken to evaluate the microshear bond strength (µSBS) of three different adhesive systems with the plasma treatment of the dentin surface and thermocycling.
Thirty-six bovine incisor teeth were stored in 0.5 chloramine solution for one week after extraction, then stored in distilled water to prevent dehydration. Distilled water was replaced every day [13]. The time interval between tooth extraction and the study was three months. Then all the teeth were decoronated at the CEJ using a high-speed flat-end diamond bur (Komet USA, Rock Hill, SC, USA). The samples were mounted in uniform same-sized plastic molds measuring 3 cm in height using cold-cured acrylic resin (Acropars, Tehran, Iran), with the buccal surfaces of the samples facing upward and slightly higher than the acrylic resin surface. The samples were placed at the center of the mold base. After removing the enamel on the buccal surface with a high-speed bur, the smooth dentin surfaces on the buccal aspect were exposed with a fine diamond disk in a polishing device under a wet condition and polished with 600-grit silicon carbide paper to create a uniform smear layer (Fig. 1). The mid-buccal area dentin and the most superficial dentin (on the external third) were used in the present study because the density and diameter of dentinal tubules increased near the pulp, which might affect the capacity of the bonding system. Then the samples were evaluated under a stereomicroscope so that no enamel would be present in the bonded area.
The prepared teeth were randomly assigned to three groups in terms of the adhesive agent used. The adhesives used in the present study were as follows (Table 1):
(1) Two-step self-etch Clearfil SE bond (Kuraray, Japan).
(2) Two-step etch-and-rinse Adper Single Bond (3M ESPE, USA).
(3) Universal Bond G-Premio Bond (GC, Japan).
| Materials | Manufacturer | Composition | Batch Code |
| Clearfil SE Bond | Kuraray Medical Inc,Japan | Primer: MDP; HEMA; Hydrophilic Dimethacrylate; Camphorquinone; water. Adhesive: MDP; HEMA; Bis-GMA; Hydrophobic Dimethacrylate; N, N diethanol p-toluidine; Camphorquinone bond; Silanated colloidal silica | 1608241 |
| Filtek Z250 XT Shade:A2 | 3 M- ESPE, USA | Matrix:Bis-GMA, Bis-EMA, UDMA, CQ Filler: Zr/Si 60% V Average particle size:0.06μm | N780437 |
| G-Premio Bond | GC, Japan | MDP, 4-MET, MEPS, BHT, acetone, dimethacrylate resins, initiators, water | 160209 |
| Single Bond 2 | 3 M- ESPE, USA | Adhesive: Bis-GMA; HEMA; Dimethacrylatas; Polyalkanoic acid copolymer; initiators; water; and ethanol. | S3456 |
| Ultra-Etch | Ultradent, USA | 35% phosphoric acid Silica thickener Depth of etch 15 s = 1.5µm | UX10947 |
In the present study, an atmospheric plasma jet (Nik Fannavaran Plasma, Model: Medaion-s, Iran) was used at 8 mA level and argon gas with a flow rate of 3000 standard cubic centimeters minute (sccm) with a 5-mm distance between the dentin and the instrument’s nozzle. Based on previous studies, a 30-second plasma application is necessary to achieve the highest level of bonding surface between the adhesive and dentin [14, 15].
The plasma gas temperature remains <37ºC as long as the flow rate is 3000 sccm [15]. After this stage, the three adhesives were applied separately on the surface of prepared dentin surfaces according to the manufacturer’s instructions [16].
2.1. Application of the Materials
The materials in the three study groups were applied to the samples according to the manufacturer’s instructions (Table 2). An LED light-curing unit (Bluephase® Style, Ivoclar Vivadent, Schaan, Liechtenstein) was used to cure the samples at a light intensity of 1200 mW/cm2.
After curing the bonding agents for 20 seconds, for each sample, hollow cylinders were cut from a Tygon tube (TYGON® R-3603 Laboratory Tubing, Gobain Performance Plastic, Maine Lakers, FL, USA), measuring 3 mm in height and 1 mm in internal diameter, and placed on the prepared surface. After curing with an LED light-curing unit (Bluephase® Style, Ivoclar Vivadent, Schaan, Liechtenstein) at a light intensity of 1200 mW/cm2, a nanohybrid composite resin (XT Z250, TM, ESPE, shade A2) was accurately placed into each tube using a Dycal instrument and light-cured for 40 seconds according to the manufacturer’s instructions. Previous studies have shown that the composite resin type does not affect the bond strength, and the composite resin selection is not important [17].
In all three groups, two subgroups were prepared for thermocycling according to ISO 11405 specification for testing adhesion to tooth structure before and after plasma treatment.
Then the samples were included in deionized water at 37ºC. Then the Tygon tubes were separated from the composite resin samples using a #12 scalpel blade. The samples were divided into two subgroups in terms of the test time: one group was subjected to the µSBS test immediately after being separated from the Tygon tubes. However, the samples in the other subgroups were subjected to artificial aging using a 5000-round thermocycling procedure in a thermocycling unit at 5±2ºC and 55±2ºC. Each cycle consisted of three rounds, with immersion in warm water for 20 seconds, immersion in cold water for 20 seconds, and a transfer time of 10 seconds [18, 19].
After the samples underwent a digital measurement procedure using a digital instrument, the samples were tested in a universal testing machine (Zwick/Roell) at a strain rate of 1 mm/min.
This device has a jaw in which the samples were placed vertically. Several loops were prepared from an orthodontic ligature wire, whose one head was placed around the samples, with the other end placed around the device rod. Then the samples were pulled until they were debonded. The shearing force was recorded in Newton, which was converted to MPA by dividing it to the surface area of the composite resin cylinder (i.e., 1 mm2):
| Material | Application Technique | Application Procedure |
| Clearfil SE Bond | Two-step self-etch | First, the self-etch primer was applied to the dentin surface with a microbrush for 20 seconds and dried with a compressed air current. Then, a layer of SE bond adhesive was applied on dentin, dried with an air current, and light-cured for 10 seconds. |
| Adper Single Bond | Two-step etch and rinse | First, the dentin was etched with 37% phosphoric acid gel for 15 seconds. The dentin surface was then rinsed with copious amounts of water for 10 seconds to eliminate the etching gel, and the adhesive was scrubbed on the wet dentin surface with a microbrush for 20 seconds and dried with a mild current of air for 5 seconds. Finally, the second layer of the adhesive was applied, gently dried with the air syringe, and light-cured for 10 seconds. |
| G-Premio Bond | One-step self-etch | The adhesive was scrubbed with a microbrush for 20 seconds. |
2.2. Preparation of the Samples for SEM Evaluation
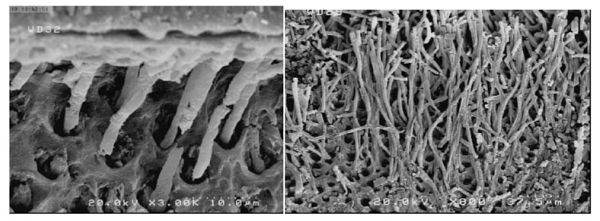
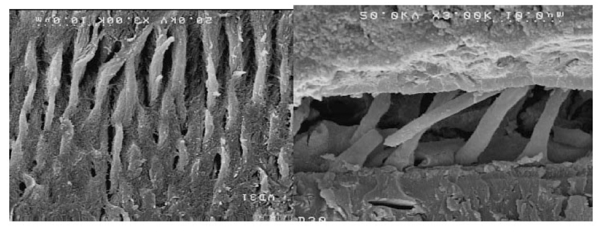
Scanning electron microscopy (FESEM, nova nanosem450, Sydney) was applied to evaluate the dentin-adhesive interface and resin tags. The plasma-treated and non-plasma-treated dentin samples underwent acid bleaching procedures after cross-sectioning to eliminate the organic and inorganic dentinal tissues [20, 21].
First, the dentin samples were immersed in 5M hydrochloric acid and 5% NaOCl solution after rinsing in distilled water for 30 seconds. Then after rinsing in distilled water for dehydrating the dentin surface, the samples were immersed in a mixture of water and ethanol with increasing concentrations of ethanol (50%, 70%, and 80%) for 15 minutes in each mixture, followed by immersion in 95-100% ethanol for 30 minutes. After drying, the samples were placed on an aluminum stud for coating with 5-nm gold particles (Desk Sputter Coater, DSR1). The samples were evaluated under a scanning electron microscope at 5 kV at ×800 and ×3000 magnifications.
2.3. Statistical Analysis
Three-way ANOVA was used to evaluate the effect of adhesive, plasma application, and thermocycling. Post hoc Tukey tests were used for two-by-two comparisons of μSBS between the groups. Statistical significance was set at P<0.05. (Chart. 1)
3. RESULTS
Three-way ANOVA showed that the triple interventions of surface plasma treatment, different adhesives, and thermocycling were significant (P<0.005). Post hoc tests were used due to the significant results of ANOVA.
3.1. The Effect of Plasma on the Bond Strength of Different Adhesive Systems without Thermocycling
The application of plasma without thermocycling has a non-significant negative effect on the bond strength in the Adper Single Bond (P=0.44). However, in the Clearfil SE Bond group and G-Premio group, plasma has positively influenced microtensile bond strength significantly (P<0.001).
Two-by-two comparisons of the groups with post hoc Tukey tests after surface preparation with plasma without thermocycling showed that the bond strength of Clearfil SE adhesive after plasma application was significantly higher than that of the Adper Single Bond adhesive (P<0.001). The bond strength of Clearfil SE adhesive after plasma application without thermocycling was significantly less than that of G-Premio adhesive (P=0.046). The bond strength of Adper Single Bond adhesive after plasma application was significantly less than that of G-Premio adhesive (P<0.001) (Table 3).
| Adhesive | Max | Min | Mean |
| Clearfil SE Bond | 57.49 | 19.28 | 33.65±11.4 |
| Adper Single Bond 2 | 16.16 | 6.96 | 11.39±2.7 |
| G-Premio Bond | 58.82 | 24.35 | 44.41±9.7 |
3.2. The Effect of Thermocycling on Different Adhesive Systems with Plasma Treatment
Thermocycling has a significantly negative effect on the bond strength of the Adper Single Bond group (P=0.008); however, thermocycling has no significant effect in the two other groups, Clearfil SE (P=0.96) and G-Premio (P=0.92).
Two-by-two comparisons of the adhesive groups with plasma application and thermocycling with post hoc Tukey tests showed that the bond strength of Clearfil SE adhesive after plasma application and thermocycling was significantly higher than that of Adper single bond (P<0.001). The bond strength of G-Premio adhesive after plasma application and thermocycling was significantly higher than that of Adper Single Bond adhesive (P<0.001) (Table 4).
| Adhesive | Max | Min | Mean |
| Clearfil SE Bond | 54.96 | 19.61 | 33.46±8.5 |
| Adper Single Bond 2 | 11.59 | 2.03 | 7.91±3.05 |
| G-Premio Bond | 59.88 | 25.57 | 39.08±8.21 |
3.3. The Effect of Thermocycling on Different Adhesive Systems without Plasma Treatment
Thermocycling without plasma application decreased the bond strength in the Adper Single Bond (P=0.054) and increased it in Clearfil SE groups (P<0.001) significantly, with no significant increase in bond strength for the G-Premio group (P=0.92) (Tables 5 and 6)
Table 5. A descriptive comparison of microtensile bond strength between the adhesive groups without plasma preparation and with thermo cycling.
| Adhesive | Max | Min | Mean |
| Clearfil SE Bond | 40.96 | 19.7 | 29.04±7.22 |
| Adper Single Bond 2 | 19.74 | 5.91 | 12.44±3.7 |
| G-Premio Bond | 57.26 | 18.62 | 33.34±12.92 |
Two-by-two comparisons of the adhesive groups without plasma preparation and thermocycling showed that the bond strength of Clearfil SE adhesive and Adper Single Bond adhesive has no significant difference (P>0.05). Under this condition, the bond strength of Adper Single Bond and Clearfil SE adhesive was found to be significantly less than that of G-Premio adhesive (P<0.001).
| Adhesive | Max | Min | Mean |
| Clearfil SE Bond | 25.17 | 11.13 | 18.09±3.8 |
| Adper Single Bond 2 | 36.22 | 8.56 | 18.55±9.67 |
| G-Premio Bond | 57.28 | 12.19 | 32.80±14.39 |
Among the adhesives tested, after thermocycling without plasma application, the bond strength was significantly different between the adhesives (P<0.005), with the highest microtensile bond strength in the G-Premio group (33.34±14.39 MPa), which was significantly higher than the Clearfil SE group (P=0.002) and Adper Single Bond (P=0.003).
3.4. SEM Observation
SEM evaluations showed that plasma application improved adhesive penetration and the formation of abundant resin tags with lateral ramifications that formed many anastomoses with each other (Figs. 2-5).

4. DISCUSSION
In restorative dentistry, the high bond strength between the tooth surface and restorative material decreases recurrent caries, tooth sensitivity, and marginal discoloration. Currently, dental caries are among the epidemiological and economic problems in developing and developed countries [21, 22].
The use of plasma is a fast and environmentally safe method to clean surfaces [10]. Besides, plasma use widens the orifices of dentinal tubules and decreases the thickness of the smear layer [22]. The aim of applying non-thermal plasma technology is to improve the bond strength to enamel and dentin, increasing the surface energy to allow constructive interactions between the adhesive and substrate without increasing the surface temperature [6]. In the present study, argon was used because previous studies have shown that argon creates a hydrophilic surface more effectively than helium [23]. Non-thermal plasma increases surface energy and solid surface hydrophilicity by removing hydrocarbon groups and creating hydroxyl groups, allowing deeper penetration of adhesive monomers [6].
The non-thermal plasma that leads to the deeper penetration of adhesives can be explained as follows. The plasma gas contains atoms and species of excited ions whose behavior is like a molecular sandblast, and the application of NT-APP to the demineralized dentin improves adhesive penetration [13, 15].

Various studies have shown that when non-thermal plasma is applied to the dentin surface, the penetration of the adhesives, especially those contained HEMA that are hydrophilic, increases due to increased surface hydrophilicity [20].
The improved hydrophilicity effect of the dentin surface by interacting with hydrophilic adhesive increases the penetration of HEMA. However, a light confrontation of HEMA on the adhesive and dentin surfaces results in the negative mechanical properties of the adhesive, making the interface susceptible to hydrophilic degradation. Therefore, an increase in the concentration of HEMA is not favorable in the composition of adhesives [24].
Increased penetration of hydrophilic monomers, such as Bis-GMA, is necessary to increase the bond strength and adhesion. After plasma application, excess water is eliminated from the collagen network without collapsing the collagen matrix, which increases the penetration of Bis-GMA and Bis-GMA content in the hybrid layer. On the other hand, an increase in the Bis-GMA content in the hybrid layer leads to fully formed resin tags, increasing the bond strength [16]. Besides, the availability of charged and reactive particles in the plasma induces polymerization and interaction between the dentin surface and infiltration of the adhesive [16].
Collagen fibers are natural polymers that might have functional groups embedded in their structure, protected against interaction with dental adhesives, preventing the collagen fibers from forming covalent bonds with the functional monomers of adhesives [15]. The application of plasma on the collagen surface allows collagen functional groups to temporarily lose their covers to interact with the functional groups of adhesives [15].
In the present study, a 30-second optimal application time was implemented so that the surface would have functional groups to increase bonding, with a detrimental effect on collagen fibers, according to a study by Ritts et al. [15].
The results of the present study concerning increased bond strength after plasma application are consistent with a study by Dong et al. [10], which showed that plasma application increased the bond strength of one-step self-etch systems. Besides, this study is consistent with the findings of Ayres et al. [7], demonstrating that plasma application increased the bond strength of two-step self-etch adhesives of Clearfil SE and Scotchbond Universal. However, after storage in water for a year, the bond strength decreased only in the Clearfil SE group. In the present study, the bond strength of one-step adhesives was influenced in a good way and did not decrease after thermocycling.
After the application of plasma in the one-step adhesive (G-Premio Bond), the bond strength was 44.4 MPa after 24 hours and 39 MPa after thermocycling. The presence of three different functional monomers, absence of HEMA monomer in the adhesive, applying plasma, functionalizing the surface, and increasing carboxyl groups increase surface hydrophilicity that increases monomer penetration due to the carboxyl groups in the 4MET acidic monomer resulting in bonding to collagen in addition to calcium ions simultaneously. All the factors above might explain increased bond strength after applying plasma and a lack of bond hydrolysis after plasma application. Therefore, the all-in-one G-Premio adhesive exhibited a favorable bond to dentin, consistent with previous studies [25]. Based on the results of mechanical tests and SEM evaluations, the use of plasma with the G-Premio adhesive systems significantly increased adhesive penetration and bond strength.
In contrast, the Clearfil SE bond has 10 MDP functional monomers that can form ionic bonds with the calcium in the tooth structure [26, 27]. The bond strength yielded by the bond strength test of Clearfil SE adhesive system in the present study was 29.04±7.22 after 24 hours, decreasing to 18.09±3.8 MPa after thermocycling, which was significant, contrary to previous studies, which reported that MDP showed a stable bond with calcium and hydroxyapatite [28].
After applying plasma, the bond strength of the Clearfil SE adhesive system was 33.65±7.22 MPa after 24 hours, followed by 33.46 MPa after thermocycling, indicating that the increase in bond strength was not significant compared to the G-Premio adhesive groups. This lack of significant difference might be attributed to the presence of three different functional monomers in the structure of the G-Premio adhesive system, which effectively forms bonds between the calcium hydroxide groups.
Although two-step etch-and-rinse systems were marketed to decrease the clinical steps and facilitate the procedure, they have problems, such as the simultaneous presence of hydrophilic and hydrophilic compounds in one pack, the inability of the hydrophobic monomer to penetrate the hydrophilic dentin, inability to control the thickness of dentin collagens that are exposed after applying phosphoric acid and hybridized by the adhesive agent, and the inability to form a homogeneous hybrid layer. On the other hand, matrix metalloproteinases are activated after applying phosphoric acid, decreasing the bond strength after 24 hours and after thermocycling [29].
Comparison of self-etch systems and Adper Single Bond 2, as an etch-and-rinse system, showed that self-etch systems have a higher bond strength, which was consistent with previous studies [30]. Self-etch bonding agents have functional monomers that establish chemical bonds with the calcium and phosphorus in tooth structure in the self-etch mode, resulting in higher bond strength than the etch-and-rinse systems.
Application of plasma in the etch-and-rinse adhesive groups exerted a negative effect on the bond strength. Even though it was not significant, it is different from previous studies. Studies by Kim et al. [16], Dong et al. [21], and Ritts et al. [15] showed an increase in the bond strength with two-step etch-and-rinse systems, which is different from the present study, indicating that plasma application was not effective in increasing the bond strength of two-step etch-and-rinse systems.
Rewetting after plasma application in different studies has been considered necessary due to the philosophy of wet bonding in the etch-and-rinse systems [13, 15, 21]. This procedure is contrary to the method used in the present study and its results. Kim et al. showed that the bond strength to plasma-dry groups [i.e., no rewetting on the dentin surface after plasma application] was significantly higher [16].
Kim et al. reported an increase in the penetration of Bis-GMA adhesives, increasing the bond strength to dentin after application of plasma-drying, which is different from the present study [16].
The decrease in the bond strength of two-step etch-and-rinse adhesive systems after plasma application might be attributed to the use of helium plasma in the study by Kim et al., while argon plasma was used in the present study. Another reason might be not using the rewetting procedure according to the wet bonding philosophy because after acid etching, the sensitivity of dentin to the water content between the collagen fibers increases, and the effect of plasma-drying with argon results in collagen fiber collapse, with no increase in bond strength. It might also be attributed to the mechanism of action of plasma in creating functional groups because, despite the manufacturer’s claims concerning the presence of polyalkenoic copolymer functional monomers in the Single Bond 2 adhesive system, different studies have shown the inadequacy of this monomer. It has been demonstrated that this monomer is not dissolved in an adhesive solvent, resulting in the separation of different phases in the adhesive [31]. On the other hand, studies have shown that in the adhesive systems with an ethanolic solvent, such as Single Bond 2, the functional monomers are inactivated due to esterification reaction. Adper Single Bond 2 exhibited the least microtensile bond strength after plasma application in the present study. Furthermore, the bond strength in the two-step etch-and-rinse adhesive systems without plasma application was 12.44±3.7 MPa, consistent with previous studies [30].
Finally, it might be suggested that further studies are necessary on the use of plasma with different gases [helium, oxygen, etc.] to identify the best method for surface preparation. Evaluation of surface energy changes and surface roughness in surface preparation with plasma might solve many problems in this field. Besides, prospective clinical studies might be beneficial for the use of plasma with long-term follow-up periods.
CONCLUSION
Under the limitations of the present study, it might be concluded that plasma application might increase penetration of adhesive and increase the bond strength of one-step self-etch adhesives.
In 2-step etch and rinse group, plasma application has a significantly negative effect on microtensile bond strength in the thermocycling group, but in the non-thermocycling group, the effect was not significant.
In 2-step self-etch group, plasma has positively influenced microtensile bond strength significantly in non-thermocycling group; however, thermocycling has no significant effect on microtensile bond strength.
In one-step self-etch adhesives, plasma application without thermocycling has a significantly positive effect on the bond strength, but the effect was not significant after thermocycling.
ETHICALS APPROVAL AND CONSENT TO PARTICIPATE
The Institutional Ethics Committee of Tehran University of Medical Sciences, School of Dentistry (IR.TUMS.REC.1397.017) approved the protocol for this study.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the corresponding author, [S.H], upon reasonable request.
FUNDING
This study was supported by the Tehran University of Medical Sciences, School of Dentistry, Tehran, Iran.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Mohamad-Javad Kharazifard for performing a statistical analysis of this article.


