All published articles of this journal are available on ScienceDirect.
Quality of Life in Fibromyalgia Patients with Craniomandibular Disorders
Abstract
Fibromyalgia (FM) is a rheumatic disease which affects fibrous tissues and muscles; it is characterized by chronic pain and it is often associated with craniomandibular disorders (CMD). 31 patients were assessed from March 2012 to October 2012 through the administration of specific questionnaires and following neurologic and gnatologic assessment. A relevant corre-lation between FM and CMD emerges from the present study, as 80.6% of our patients report CMD symptoms with high prevalence of myofascial pain (84%). Multivariate regression analysis revealed that the patients in the present study did not differ in score of quality of life questionnaires from patients with fibromyalgia. The neuropathic pain diagnostic question-naire (DN4) scores were positively affected by belonging to group II of Research Diagnostic Criteria of Temporomandibular Disorders (RDC/ TDM) classification, suggesting the possibility of a neuropathic component in chronic pain in this CMD group, as already speculated in our study on the correlation between burning mouth syndrome and CMD and by other au-thors in studies on chronic low back pain. However, further clinic and instrumental studies are needed in order to test this as-sumption.
INTRODUCTION
Fibromyalgia (FM), according to the American College of Rheumatology (ACR) [1] “is a chronic musculoskeletal pain syndrome of unknown etiology, characterized by widespread pain for more than 3 months and tenderness in at least 11 tender point sites out of 18”.
Other symptoms are physical and mental tiredness, insomnia and neuropsychological problems[1].
FM is a common disease with a prevalence of about 2%, that affects women more than men (3.4% versus 0.5%) [2, 3].
The causes of FM are unknown, although it is generally agreed that the persistence of musculoskeletal pain is due to the so called ‘mechanism of central sensitization’ [4-6]. De Tommaso [7] defined central sensitization as “a phenomenon present in any type of pain, nociceptive or neuropathic, where a noxious stimulus, able to recruit the C and A-delta sensory fibers, reduces in a short times the pain threshold in the primarily involved areas, a phenomenon known as hyperalgesia, while in the adjacent zones any mechanical input becomes painful, as an effect of the so-called “allodynia”.
Craniomandibular disorders (CMD) display a wide range of medical cases concerning masticatory muscles, temporomandibular joints or both. The patient affected by craniomandibular disorders can suffer pain in every component of the oro-facial district (pre-auricular zone, masticatory
muscles, temporomandibular joint) and presents a limited mouth opening with or without latero-deviation and joint noise [8].
CMD are widespread in the population: prevalence studies have reported that approximately 33% of the population have CMD signs and 75% have CMD symptoms [8, 9].
Dworkin e LeResche [10] Research Diagnostic Criteria of Temporomandibular Disorders (RDC/ TDM) classified craniomandibular disorders into three subgroups:
Group 1:
- 1a: myofascial pain;
- 1b: myofascial pain with limited opening;
Group 2:
- 2a: disk displacement with reduction;
- 2b: disk displacement without reduction, with limited opening;
- 2c: disk displacement without reduction without limited opening;
Group 3:
- 3a: arthralgia;
- 3b: osteoarthritis;
- 3c: osteoarthrosis.
CMD and FM were once considered unrelated entities, but several studies [11-15] highlighted oro-facial symptoms in patients affected by FM indicating that there could be a correlation between these two conditions [16].
Myofascial face pain, for example, was considered to be a regional expression of fibromyalgia [17]. Yunus [18] classified fibromyalgia, irritable bowel syndrome, myogenic temporomandibular disorders, tension-type headache, migraine and other chronic syndromes under the name “Central Sensitivity Syndromes (CSS)” characterized by the same underlying pathophysiology, i.e. a prolonged central sensitization [6].
The aim of our work was to determine the prevalence of CMD among patients with FM and CMD, classifying craniomandibular symptoms within the Dworkin and LeResche’s subgroups. FM syndrome and CMD are both chronic diseases and different authors studied the effects of chronic pain on the quality of life among patients affected by this [19, 20].
Gijsen et al. [21] claimed that “longitudinal studies suggest that the cumulative effect of comorbid conditions is not simply additive: certain combinations of diseases may have a greater effect on quality of life than others”. Therefore the second aim of our study was to determine the burden of CMD symptoms on quality of life among FM patients, testing the hypothesis according to which fibromyalgia subjects affected by craniomandibular disorders have poorer quality of life than fibromyalgia patient without CMD.
MATERIALS AND METHODS
This study was completed between March and October 2012 at the Neurophysiopathology of Pain Unit, Bari University Hospital. All the participants filled in an informed consent form; the Ethics Committee of the Policlinico General Hospital approved this study. Of the 100 patients examined in the above mentioned facility, reporting chronic pain not ascribable to any pathology, 55 received a fibromyalgia diagnosis. Of these 55 patients, 31 were willing to undergo a gnatologic visit and to fill in questionnaires aiming at the assessment of their quality of life (Fig. 1).
Diagram of Materials and methods section.
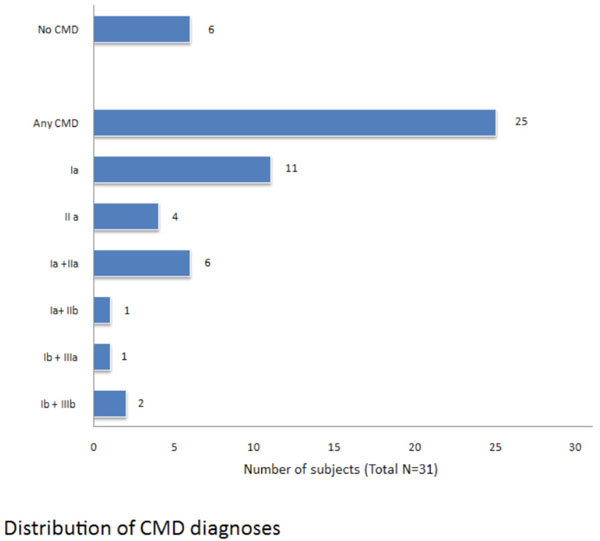
Distribution of CMD diagnoses.
Clinical-gnatologic Assessment
For each sample component age, gender and profession. pain severity and frequency information were measured and reported. A neurological examination was carried out followed by a clinical-gnatologic assessment verifying the presence of widespread pain also on the orofacial region level and the presence of CMD signs and symptoms. In the first examination, the quality of life of fibromyalgia patients was measured using various instruments.
Neurological Examination
Patients were submitted to a standardized manual tender point survey [22]: “tender point count was defined as the number of positive tender points” (tender pn), while “total tender point score was calculated by adding individual scores for all 18 tender points”(tender p tot).
At the end patients answered a neuropathic pain diagnostic questionnaire (DN4), a validated tool to identify neuropathic pain on a score equalling ≥4 out of 10 [23].
Clinical-gnatologic Assessment
All fibromyalgia patients underwent a visit of the orofacial district according to the RDC/TMD guidelines [10].
Orofacial pain was measured with a ten centimetres visual analogue scale (VAS).
Presence of oral parafunctions like bruxism, clenching, lip and cheek biting etc was also observed.
Quality of Life Assessment
Quality of life was measured by means of the following instruments: Medical Outcome Short Form Health Survey (SF-36) and the Fibromyalgia Impact Questionnaire (FIQ).
SF-36 is a self-administered questionnaire composed of 36 questions regarding eight health concepts: physical functioning, physical role limitations, emotional role limitations, bodily pain, general health perceptions, vitality, social functioning and general mental health [24].
The answers to questionnaires lead, summing all values, to two measures: SF-36 ISF which includes the values regarding difficulties in physical activity and SF-36 ISM which includes the values about psychological and mental components. Higher scores represent a better functioning [19].
FIQ, according to Burckardt et al. [25], “is a self-reported instrument that measures the current health status of the FM patient”; it includes 13 items assessing the difficulty in performing common, everyday life activities, general health, working activity and 7 items assessing the intensity/severity of symptom on a VAS scale from 0 to10.
The final score may vary from a minimum of 0 to a maximum of 100 (higher scores represent a higher impact of the disease on the patient’s quality of life) [25].
In the present study we adopted a 0-70 simplified score system that includes only 7 items assessing the intensity/severity of symptom (with 70 indicating greater impairment).
Statistical Analysis
For each of the selected patients a file was filled in, it included demographic variables, pain duration at t0, psychological tests (SF-36 ISF, SF-36 ISM, FIQ), VAS score, DN 4 Questionnaire outcomes, number of tender points, pain frequency and location, parafunctions, CMD group to which the patient was assigned in accordance with the RDC/TDM classification. The files were then put in a database created with File Marker Pro software and analyzed with STATA MP11 software. The quantitative variables were expressed as means ± standard deviation; qualitative variables as proportions. As to assess the effect of age, gender, pain duration at t0, VAS, tender point, pain frequency and location, presence of parafunctions, CMD group on the outcomes of psychometric tests about the quality of life, multivariate regression models were set. For all the used tests a value of P < 0.05 was considered substantial.
RESULTS
We studied 31 patients, 3 males (9.7%) and 28 females (90.3%), their age ranged from 20 to 65 years (average age 47.9 ± 9.9 years).
The average pain duration, at the time patients were hired, was 8.1 ± 8.0 months. 18 patients (58.1%) reported a daily pain frequency, 11 (35.5%) a weekly frequency and 2 (6.4%) a monthly frequency.
In 25 FM patients (80.6%) symptoms and signs of craniomandibular disorders were observed.
18 patients (58.1%) had parafunctions, more in detail, 12 (38.7%) were affected by bruxism, 6 of whom (19.3%) used the bite.
In Fig. (2) we reported the distribution of craniomandibular disorders diagnosis according the RDC/TMD classification Summarizing, a muscular disorder is present in 21 patients (84%), a disc dislocation in 11 patients (44%), inflammatory and degenerative joint disorders in 3 patients (12%). In 40% of cases we have a combined diagnosis.
Descriptive statistics were performed to summarize sample characteristics in Table 1.
Sample characteristics.
| Evaluation | Mean | Standard deviation |
|---|---|---|
| DN4 | 4.9 | 2.6 |
| VAS | 7.9 | 1.8 |
| Tender pn | 15.3 | 4.3 |
| Tender p tot | 94.0 | 43.4 |
| SF-36 ISF | 29.8 | 8.5 |
| SF-36 ISM | 37.4 | 11.8 |
| FIQ | 54.2 | 13.9 |
Results of comparison between FM patients with CMD and FM subjects without any symptoms Differences between groups are significant at p<0.05. N.S.: Not significative.
| Characteristic, means (SD) | FM patients with CMD (N=25) | FM patients without CMD (N=6) |
|---|---|---|
| SF-36 ISF | 29.8±8.9 N.S. | 29.5±6.9 |
| SF-36 IFM | 36±10.5 N.S. | 43.1±16.1 |
| FIQ | 54±14.3 N.S. | 53.1±12.2 |
In Table 2 are presented the results of a comparison of quality of life assessment between FM patients affected by craniomandibular disorders and FM subjects without any symptoms.
As we note, quality of life scores for all domains of the SF-36 were similar between patients groups.
Multivariate regression estimates that FIQ scale scores are not affected by the presence of temporomandibular disorders but by the tender point total score (coefficient 0.21; t=2.5; p=0.03). DN4 questionnaires scores are negatively affected by the presence of parafunctions (coefficient = -3.7; t=2.38; p=0,038) and by the belonging to group I of RDC/TDM classification; whereas the above mentioned scores are positively affected by the belonging to group II (coef 3.2; t=3.27; p=0.008).
DISCUSSION
Our results suggest that craniomandibular disorders are pervasive among the FM population, as 80.6% of our patients report CMD symptoms with high prevalence of myofascial pain (84%), alone or associated to disc dislocation or to arthralgia. These results are consistent with Plesh et al. [11] who reported that 75% of subjects with FM suffered from muscle-related CMD.
Myofascial pain in the masticatory muscles is a significant component in fibromyalgia according several authors.
Hedenberg-Magnusson et al.[26] in their study found that 94% of 191 patients with FM also displayed symptoms of CMD. Fricton [14] in 2004 speculated that “clinical overlap between these conditions may reflect a shared underlying pathophysiologic basis” [14, 15]. Woolf [6] and Yunus [18] defined FM and myogenic temporomandibular disorders as “overlapping conditions” that are, together other diseases, “collectively known as central sensitivity syndromes”.
According to these authors, there is a strong evidence of central sensitization in myofascial pain, “including decreased pain threshold by various nociceptive stimuli at sites remote from painful areas, accentuated nociceptive spinal flexion reflex and augmented cortical activation by functional magnetic resonance imaging” [18].
An important consequence of this is relevant to the treatment of these pathologies: “since total disease burden with functional impairment is greater in patients with many associated conditions, a practicing physician should treat these conditions for optimal results” [27, 28].
The second aim of our study was to determine the burden of CMD symptoms on quality of life among patients with FM.
Quality of life has been described by the World Health Organization as “an individual’s perception of their position in life in the context of the culture and value system of which they live with the relation to their goals, expectations, standards and concerns”[29].
Several studies suggest that certain combinations of comorbid conditions may have a greater effect on quality of life than others [21]. In our study, the quality of life scores were similar in fibromyalgia patients with or without CMD: FM totally contributed to the physical and mental disability in affected individuals that we examined. Also Marcus et al. [30] in their epidemiological study on fibromyalgia and headache found that migraine did not affect the perception fibromyalgic patients had of their disease. Hoffman and Dukes [31], in an article on the health status of patients with fibromyalgia examined the extent to which FM contributed to the overall health status burden of patients with and without a specific concurrent condition and they found that “while health status in general population deteriorated in conjunction with the number of pain condition present, FM had a remarkably consistent pattern of health status impairment even that it occurred alone”.
In other words, patients with FM are already so burdened by their condition that the introduction of an additional pain condition (as masticatory muscle pain in our patients) does not affect their health related quality of life (HRQoL) [32].
Multivariate regression analysis revealed that the DN4 questionnaires score are positively affected by the belonging to group II of RDC/TMD classification. DN4 questionnaire is a validated tool to identify neuropathic pain and this suggests the possibility of a neuropathic component in chronic pain in this CMD group (disk displacement with and without limited opening) as already speculated in chronic low back pain [33, 34] and in our study on the correlation between BMS and TMD [35].
Limitations of this study are the small sample size and that all health status data were based on self-report. Further clinic and instrumental analysis (such as a standardized protocol of quantitative sensory testing and laser-evoked potentials) are needed in order to test the hypothesis of a neuropathic component of pain due to craniomandibular disorders.
CONCLUSION
The results of the present study suggest the existence of a close association between FM and CMD. A high percentage of FM patients (80.6%) indeed resulted to be positive to at least one RDC/TMD diagnosis.
We therefore suggest to subject FM patients to a gnatologic assessment in order to complete their diagnostic situation.
On the other hand the dentist should be able to perform a differential diagnosis between localized or systemic muscle disorders, as to better handle patients with orofacial pain. In fact, once identified, TMD patients who have co-morbid conditions such as fibromyalgia, can best be treated by an interdisciplinary team of clinicians.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


