Salivary 8-OHdG Induction by Physical Exercise Training Under Food Restriction
Abstract
Objective:
We previously reported that physical exercise under food restriction induced alterations in saliva secretion, including a decrease in salivary kallikrein activity, while exercise training alone did not affect the overall saliva content. The objective of the present study was to examine the involvement of oxidative stress in alterations of salivary secretion due to physical exercise under food restriction.
Methods:
Male ICR mice at 32 weeks of age weighing from 39 to 50 g were divided into three groups: an exercise group with food restriction (EXP), an exercise group without food restriction (EXA) and a control group (CTL). The EXP group was fed the same amount of diet as the CTL group (pair-feeding). The EXP and EXA groups had access to a “voluntary running wheel” for exercise. The pilocarpine-stimulated whole saliva was collected from the oral cavity by micro-pipette over 15 min for 12 weeks after the beginning of the experiment. The salivary and serum 8-Hydroxy-2’-deoxyguanosine (8-OHdG) were determined.
Results:
The salivary 8-OHdG levels in the EXP group were significantly higher than in the CTL and EXA groups (p<0.01).
Conclusion:
The alterations of salivary secretion in mice undergoing chronic exercise training under a food-restricted diet may occur as a result of oxidative stress.
1. INTRODUCTION
Moderate physical activity has a beneficial effect on the maintenance of health and the prevention of disease [1]. On the other hand, extreme or overly intense physical training appears to have an adverse effect on overall health. Over-training induces oxidative damage to DNA in the liver [2], and leads to a suppression of mucosal immunity [3]. There are many kinds of sports that require athletes to undergo dietary restriction in addition to intense physical exercise, such as wrestling and boxing. The athletes may therefore be inferred to be under physical and mental stress. A previous experimental study demonstrated that physical exercise in mice under dietary control induced alterations in saliva secretion, while exercise training alone did not affect the saliva contents [4]. The total protein secretion and kallikrein activity decreased by 20–30% in the physical exercise under dietary-controlled mice between 4 and 12 weeks after the start of the experiments in comparison to the control mice, thus suggesting that signal transduction in the sympathetic nervous system was downregulated, since kallikrein is secreted in response to alpha1-adrenergic receptor stimulation [5]. The study suggested that the salivary function was affected by chronic physical and/or mental stress.
The oxidative stress is related to various mechanisms such as apoptosis, cancer progression inflammation and aging. Concerning salivary gland disease, salivary antioxidants are increased in rheumatoid arthritis patients [6]. The levels of 8-hydroxy-2'-deoxyguanosine (8-OHdG) and hexanoyl-lysine (HEL) have been shown to increase in the saliva of patients with Sjögren’s syndrome, an autoimmune disorder in which immune cells attack and destroy the exocrine glands, and the level of mitochondrial glutamic-oxaloacetic transminase (m-GOT) is significantly correlated with 8-OHdG and HEL [7]. 8-OHdG is formed when the guanine in DNA undergoes oxidative damage by reactive oxygen species (ROS) [8, 9]. It is possible to prove cytotoxicity via oxidative stress by detecting 8-OHdG, and 8-OHdG has therefore been widely used as a biomarker of oxidative damage [10].
The purpose of the present study was to clarify the involvement of oxidative stress in alterations of saliva secretions following physical exercise in dietary-controlled mice.
2. MATERIAL AND METHODOLOGY
2.1. Animals
Thirty-two-week-old male ICR mice (Japan SLC Inc., Shizuoka, Japan), weighting from 39 to 50 g, were used in the present study. The animals were individually housed in plastic cages under regular light/dark conditions (lights on, 0800-2000 h) and the room temperature was maintained at 23 ± 1°C and the humidity was between 60 and 80%. Animals had access water ad libitum and laboratory pellets (CE-2; CLEA Japan, Inc., Tokyo, Japan) twice a day, in the morning (9:30-10:30) and evening (16:30-17:30).
2.2. Experimental Conditions
The animals were divided into three groups according to the experimental condition; an exercise with food restriction (EXP), an exercise without food restriction (EXA) and control (CTL). For exercise in the EXP and EXA groups, a “voluntary running wheel” was used. Mice were housed individually in cages equipped with a running wheel (20-cm in diameter, Shinano Co., Tokyo, Japan). Each wheel revolution was registered by a magnetic switch, which was connected to a counter. The number of revolution was recorded daily [11, 12]. The EXA and CTL mice were fed ad libitum for an hour twice a day, thus the EXA mice showed more food consumption than the CTL mice. The EXP group was fed the same amount of food as the CTL (pair-feeding).
It is well established that rats fed a liquid diet exhibit a parotid gland atrophy and decrease of salivary protein secretion [13, 14]. The changes are thought to result from decreased gland function due to the elimination of the requirement for mastication of food. And then, the EXP group was fed the same amount of food as the CTL to compensate the difference of mastication.
2.3. Collection of Saliva
Saliva was stimulated by 0.2 ml of pilocarpine (0.5 mg/kg, ip injection, Wako Pure Chemical Ind., Osaka, Japan), and was collected under general anesthesia (60 mg/kg body weight of ketamine, Sankyo Co., Tokyo, Japan; and 6 mg/kg body weight of xylazine, Bayer chemicals Japan Ltd., Tokyo Japan) at 4, 8, and 12 weeks in the experiment (at the same time of the day). Saliva collection was started 5 min after pilocarpine injection. Saliva was collected from the oral cavity by micropipette for 15 min and it was placed in micro-centrifuged tubes placed in an ice bath and weighed. After collecting saliva, mice were further anaesthetised with ether and collected blood from an artery. Blood samples were centrifuged at 3,000×g for 5 min to obtain sera. Saliva and sera sample were stored at -80 C until the analyses were performed.
All experimental procedures were carried out in accordance with the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes and were approved by the Committee on Animal Care of Tsurumi University School of Dental Medicine.
2.4. Determination of Saliva and Serum 8-OHdG
Saliva and sera samples were centrifuged at 5,000×g for 5 min. A competitive enzyme-linked immunosorbent assay (ELISA) was conducted for samples in triplicate using an 8-OHdG monoclonal antibody (Institute for the Control of Aging, Shizuoka, Japan). A 50-µl aliquot of each saliva or sera supernatant samples was placed in the wells of a 96-well plate and were incubated for 1 hr. The wells were washed 3 times with a buffer solution, and 100 µl of an HRP-conjugated anti-mouse IgG antibody was added and incubated at room temperature for 1 hr. The wells were washed 3 times with wash buffer, and 100 µl of hydrogen peroxide/citric acid containing phosphate-buffered 1% 3, 3’, 5, 5’-tetramethylbenzidine was added. After 15 min, 1 M phosphate buffer was added into the well to stop the reaction. The absorbance of the reaction mixture was measured at 450 nm with a micro-plate reader (Bio-Rad Laboratories, Inc., USA), and a standard curve was used to determine the amount of 8-OHdG present in the test samples.
2.5. Statistical Analysis
All results are expressed as the mean ± SD. The statistical analyses were performed with one-way ANOVA when comparing the three experimental groups and the Mann-Whitney U test was used when the EXP and EXA groups were compared with the CTL group. P-values of less than 0.05 were considered to be statistically significant.
3. RESULTS
8-OHdG levels in the EXP saliva were significantly higher (16.33 ± 4.33 ng/ml) than in the CTL group (8.92 ± 0.77 ng/ml) and the EXA group (9.56 ± 1.82 ng/ml) (p<0.01) (Fig. 1). On the other hand, 8-OHdG levels in the sera of the EXP mice (6.59 ± 4.33 ng/ml) were not significantly different compared with the CTL (6.99 ± 0.46 ng/ml) and EXA groups (6.83 ± 0.78 ng/ml), thus suggesting that 8-OHdG was secreted from the salivary gland, but was not present in serum.
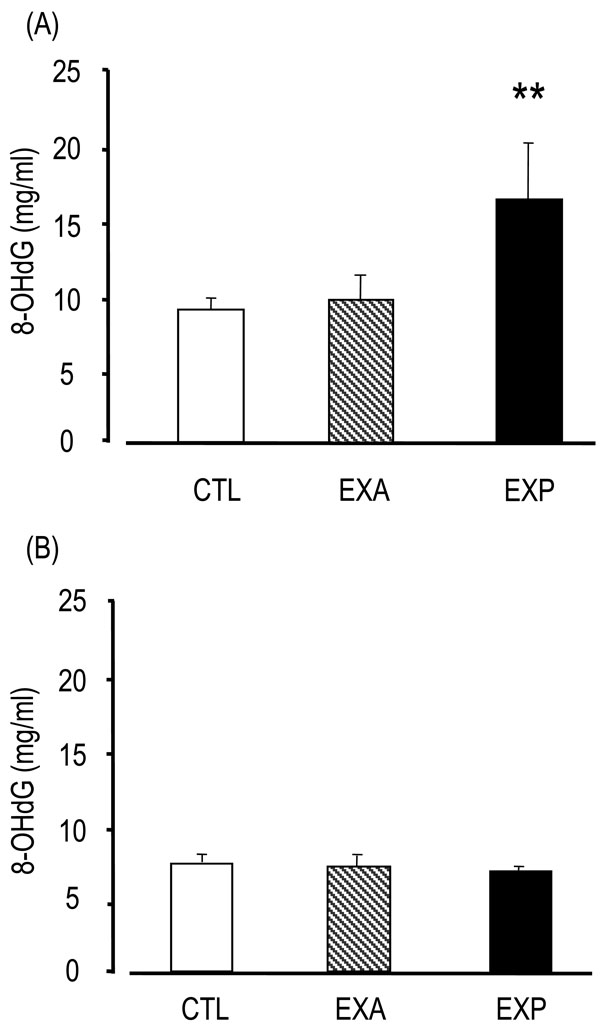
Changes of saliva and serum 8-OHdG.
Saliva is collected from the oral cavity for 15 min after the injection of pilocarpine. The 8-OHdG levels were assessed by competitive enzyme-linked immunosorbent assay (ELISA) using an 8-OHdG monoclonal antibody. The 8-OHdG levels are indicated in saliva (A) and serum (B). Values of saliva are the means ± SD of nine mice in the CTL, six mice in the EXA, five mice in the EXP. Values of serum are the means ± SD of six mice in the CTL, five mice in the EXA, five mice in the EXP. **p < 0.01, Mann-Whitney’s U-test.
4. DISCUSSION
The present study demonstrated a significant increase of the oxidative stress marker 8-OHdG in saliva in mice undergoing long-term exercise training under diet restrictions. Because there were no increases in HEL or m-GOT levels (date not shown), the guanine in DNA underwent oxidative damage by ROS without apparent occurring lipid peroxidation or cell damage. In the group of mice with long-term exercise and without food restrictions, changes in the saliva secretion and salivary protein contents were not recognized, and the level of salivary 8-OHdG was not altered. Therefore, these results suggest that an increase in oxidative stress markers in saliva is related to the alteration of salivary secretion and qualitative changes in salivary proteins.
The oxidative stress is related to various mechanisms such as apoptosis, cancer progression, inflammation and aging. There are many putative roles for nitric oxide (NO) in the salivary glands, and include ensuring an adequate blood supply during long-term salivary secretion, serving as a feedback mechanism to the periacinar neurones, regulating cell growth and differentiation in the surrounding tissue, and participating in the host defense barrier [15]. Regarding the role of NO signaling in salivary secretion, β-adrenergic receptor stimulation activates NO production via NO synthase (NOS). Both endogeneous and exogeneous NO activate cGMP synthesis and G kinase. Following activation of the NO/cGMP/G kinase pathway, the Ca2+ signal induced by stimulation of the PLC-coupled receptor, muscarinic and alpha-adrenergic receptors, is amplified, forming the basis for propagated secretion (15). On the other hand, NO has been shown to play a role in the pathogenesis of a number of oral diseases. Aberrant NO production in the acinar cells may contribute to the disease progression in Sjögren’s syndrome [15, 16]. The levels of 8-OHdG have been shown to increase in the saliva of patients with Sjögren’s syndrome [7].
Increase of salivary 8-OHdG is thought to be due oxidative damage of the salivary glands. One of the possible reasons in the increase of oxidative stress is speculated to be chronic stimulation of adrenergic receptor. Isoproterenol induces cAMP via β-adrenergic receptor stimulation, and cAMP-dependent protein kinase A (PKA), which is activated by cAMP, stimulates the secretion of salivary proteins. Isoproterenol-stimulated secretion is associated with increased oxygen and glucose consumption [17]. Lipid peroxidation in the submandibular gland is increased following a single injection of isoproterenol, and accordingly the activity of the antioxidative enzyme superoxide dismutase (SOD) is increased [18]. Reactive oxygen species (ROS) such as superoxide anion, hydroxyl radicals, and hydrogen peroxide are constantly produced in aerobic organisms. The superoxide dismutases (SOD) are antioxidative enzymes that act as a first defense against oxidative stress [18]. Long-term physical exercise under dietary control is speculated to induce physical and/or mental stress, and therefore causes chronic stimulation of the sympathetic receptors [4]. Salivary oxidants might be increased by chronic and excessive β-adrenoceptor stimulation as a result of the imbalance of oxidants and antioxidants, namely the accumulation of ROS and the anti-ROS SOD activity.
In isolated rat mesenteric arteries, selective stimulation of alpha1-adrenoceptors with phenylephrine induced matrix metalloproteinase (MMP) transactivation of the epidermal growth factor receptor (EGFR), mitochondrial ROS production and vasoconstriction [19]. The study suggests that vascular disease is established by adrenoceptor stimulation, and oxidative stress contributes to this process. The mechanism is an attractive one to explain the results of the present study. The decrease of salivary kallikrein activity in mice undergoing chronic exercise training under dietary restriction might occur as a result of ROS production.
Chronic stress is known to affect salivary gland function. Chronic exposure of rats to light promotes the degradation of parotid acini and the desensitization of submandibular gland β-adrenergic receptors [20], and alpha2-adrenergic desensitization was observed in these constant light-exposed rats as well as restraint-stress rats, in which 2 h daily immobilization was induced for 14 days [21]. Because the localization changes of these receptors following agonist stimulation are well-known phenomena, there is a possibility that desensitization occurs under stress conditions such as physical exercise training under dietary-restricted conditions. Since kallikrein is secreted in response to alpha11-adrenergic receptor stimulation [5], down regulation might be involved in the alpha1-adrenergic receptor stimulation.
The present study suggests the involvement of oxidative stress in salivary changes due to chronic stress. Therefore, further investigations into the participation of NO and ROS and their role in the intracellular signal transduction are required.
ACKNOWLEDGEMENT
Funding: This study was supported in part by a Grant-in-Aid for Scientific Research from Ministry of Education, Culture, Sports, Science and Technology of Japan.
Ethical approval: All experimental procedures were carried out in accordance with the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes and were approved by the Committee on Animal Care of Tsurumi University School of Dental Medicine.


