All published articles of this journal are available on ScienceDirect.
Effects of Clenbuterol, a β2-Adrenergic Agonist, on Sizes of Masseter, Temporalis, Digastric, and Tongue muscles
Abstract
We compared the hypertrophic effects of clenbuterol, a β2-adrenergic agonist, on the masseter, digastric, and temporalis with those on the tongue, tibialis anterior, soleus, diaphragm, and heart. The weights of masseter, digastric and temporalis in the clenbuterol group were 36 ~ 56% greater than those in the control group, whereas those of the tibialis anterior, diaphragm, and heart weights in the clenbuterol group were 9 ~ 33% greater than those in the control group. No significant difference in the weights of the soleus and tongue was found between the control and clenbuterol groups. Taken together with our present and previously reported results, it is suggested that the hypertrophic effects of clenbuterol on the masseter, digastric, and temporalis are greater than those on the limb, trunk, and heart.
INTRODUCTION
Clenbuterol [4-amino-α (t-butyl-amino) methyl-3,5-dichlorobenzyl alcohol] is a β2-adrenergic agonist and non-steroidal anabolic drug for sports doping. According to the recent World Anti-Doping Agency documents, the use of clenbuterol was the fifth most common case in the number of anabolic drugs–used contravention in 2006 (53 cases) [1]. Clenbuterol is known to induce hypertrophy of skeletal muscles such as the soleus, gastrocnemius, and extensor digitorum longus, as well as on the masseter and heart muscles [2-10]. The precise mechanism for the hypertrophy of skeletal and cardiac muscles induced by clenbuterol remains unknown. One leading hypothesis is that clenbuterol induces the hypertrophy of the skeletal muscles through the β2-adrenergic receptor by up-regulating the expression of insulin-like growth factors (IGFs) [7, 11-13] which play essential roles in the development, growth, and regeneration of skeletal muscles [14-20].
Muscle satellite cells are mononucleated and quiescent stem cells that reside between the sarcolemma and basal lamina of adult myofibers [21,22]. In response to stimuli such as mechanical loading, unloading, denervation, and injury, the satellite cells are activated through several growth factors containing IGFs and this activation is thought to induce adaptive changes of skeletal muscle such as hypertrophy, the alteration of fiber type, and regeneration [16, 21-23]. Recently, we have reported that the pool size of satellite cells in the masseter muscle of the muscle dystrophy model mouse (mdx) is greater than those of other muscles such as the gastrocnemius, soleus, and diaphragm [24] and we hypothesized that the hypertrophic effect of clenbuterol on the craniofacial muscles containing the masseter muscle is greater than on other muscles.
In the present study, to test this hypothesis, we measured the wet weights of masseter, temporalis, digastric and tongue muscles, and compared them with those of tibialis anterior, soleus, diaphragm, and heart muscles after oral administration of clenbuterol for 3 weeks. Furthermore, to exclude the possibility that clenbuterol secondarily leads to the hypertrophy of the masseter, temporalis, and digastric muscles by directly inducing the hypertrophy of the mandible and maxilla, we measured the distance between the origin and insertion of the muscles by three-dimensionally reconstructing the images of micro-computed tomography (μCT).
MATERIALS AND METHODS
Experimental Animals, Administration of Clenbuterol, and Weighing Muscles
Ten male Wistar rats were purchased from Clea Japan, Inc., (Tokyo, Japan) and fed a hard diet (CE-2; Clea Japan, Inc., Tokyo, Japan). They were divided into control and clenbuterol groups of five rats each at 8 weeks of age. We orally administered 30 μg/ml of clenbuterol (C5423; Sigma-Aldrich Fine Chemicals, St. Louis, MO, USA) to the rats in the clenbuterol group via their drinking water for 3 weeks, while pure water was given to the rats in the control group. We daily measured the weight of each rat and consumption of pure water or water containing clenbuterol for each rat to estimate the daily dose of clenbuterol. The dose of clenbuterol was approximately 4 mg/kg of body weight/day. After 3 weeks, all the animals were killed by exsanguinations under ether anesthesia. The masseter, temporalis, digastric (anterior belly), tongue, tibialis anterior, soleus, diaphragm, and heart were immediately dissected and weighed. After removing the muscles, the whole heads were frozen and stored in -30°C until subsequent μCT analysis. Experimental protocols concerning animal handling were reviewed and approved by the Institutional Animal Care Committee of Tsurumi University School of Dental Medicine.
Morphometric Analysis of Rat Head by Micro-Computed Tomography (μCT)
The distance between the origin and insertion of masseter, digastric and temporalis muscles are analyzed by three-dimensionally reconstructing the images of μCT. The whole head of the rat was transversely scanned at 750 μm intervals with a μCT system (LCT-100, ALOKA CO., LTD., Tokyo, Japan). The images at a resolution of 480 X 480 pixels were reconstructed in three directions using image analysis software (Amira 3.1, Mercury Computer Systems, San Diego, CA) and the distances between the origin and insertion of masseter, digastric and temporalis muscles on the mandible and maxilla were measured on the three-dimensional images. Fig. (1) shows the measured points for the origins and insertions of masseter, temporalis and digastric muscles on the mandible and maxilla.
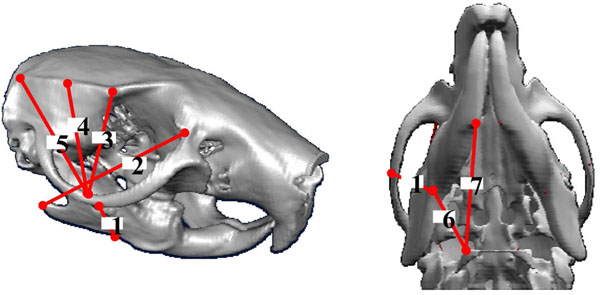
Three-dimensionally reconstructed images of mandible and maxilla by μCT showing the measured points for the origins and insertions of masseter (1, 2), temporalis (3, 4, 5), and digastric (6, 7) muscles.
RESULTS
Fig. (2) shows box and whisker plots of the body weight in the control and clenbuterol groups at 21 days of clenbuterol administration. In the plot, the dots represent the median value, the boxes represent the interquartile range, and the whiskers represent the full range of data. The median values of the body weight in the clenbuterol and control groups were 414 g and 449 g, respectively, with no statistically significant difference between the two groups.
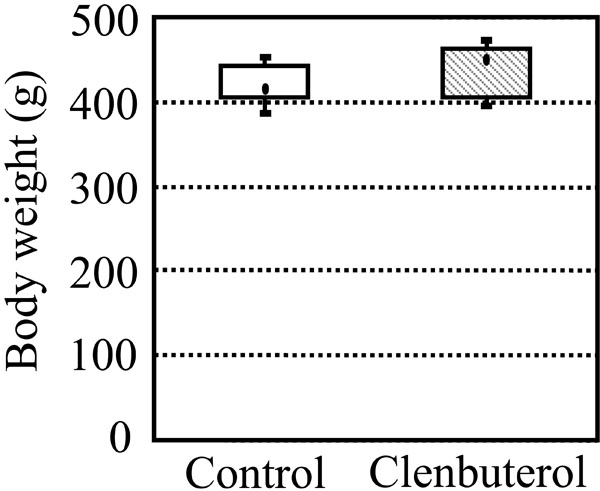
Box and whisker plot of body weights in the control and clenbuterol groups after oral administration of clenbuterol for 3 weeks. There were five rats in each group. No significant difference in the body weight was found between the control and clenbuterol groups.
Fig. (3) shows box and whisker plots of the masseter (A), digastric (B), temporalis (C), and tongue (D) weights in the control and clenbuterol groups at 21 days of clenbuterol administration. The median values of the masseter, digastric, and temporalis weights in the clenbuterol group were 1.588, 0.147, and 0.762 g, which were 46, 36, and 56% greater than those in the control group (1.091, 0.108, and 0.490 g; p<0.01), respectively. The median values of the tongue weight in the clenbuterol and control groups were 0.375 and 0.353 g, respectively, with no statistically significant difference between the two groups.
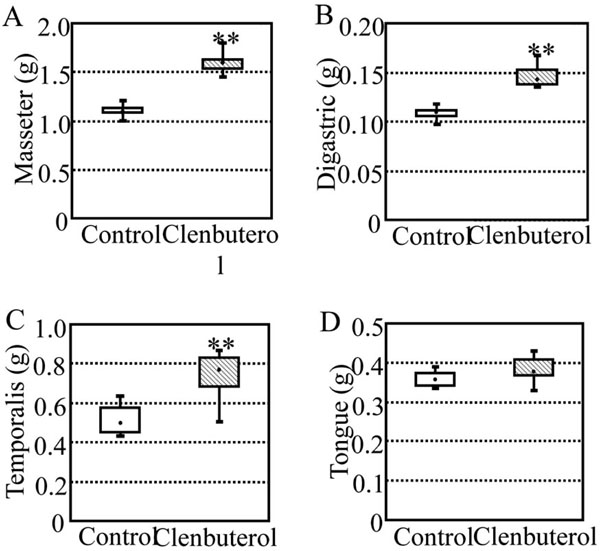
Box and whisker plots of weights of masseter (A), digastric (B), temporalis (C), and tongue (D) in the control and clenbuterol groups after oral administration of clenbuterol for 3 weeks. There were five rats in each group. Significant difference between the control and clenbuterol groups, **p<0.01. The median values of the masseter, digastric, and temporalis weights in the clenbuterol group were 46, 36, and 56% greater than those in the control group, respectively, but no significant difference in the tongue weight was found between the control and clenbuterol groups.
Fig. (4) shows box and whisker plots of the tibialis anterior (A), soleus (B), diaphragm (C), and heart (D) weights in the control and clenbuterol groups at 21 days of clenbuterol administration. The median values of the tibialis anterior, diaphragm, and heart weights in the clenbuterol group were 1.111, 1.099, and 0.923 g, which were 33, 17, and 9% greater than those in the control group (0.837, 0.937, and 0.845 g; p<0.05 ~ 0.01), respectively. The median values of the soleus weight in the clenbuterol and control groups were 0.186 and 0.218 g, respectively, with no statistically significant difference between the two groups.

Box and whisker plots of weights of tibialis anterior (A), soleus (B), diaphragm (C), and heart (D) in the control and clenbuterol groups after oral administration of clenbuterol for 3 weeks. There were five rats in each group. Significant differences between the control and clenbuterol groups: *p<0.05, **p<0.01. The median values of the tibialis anterior, diaphragm, and heart weights in the clenbuterol group were 33, 17, and 9% greater than those in the control group, respectively, but no significant difference in the soleus weight was found between the control and clenbuterol groups.
To exclude the possibility that clenbuterol secondarily leads to the hypertrophy of the masseter, temporalis, and digastric muscles by directly inducing the hypertrophy of the mandible and maxilla, we measured the distance between the origin and insertion of the muscles by μCT (Table 1) and found no statistically significant differences in these distances. This result indicates that the hypertrophic effect of clenbuterol on masseter, temporalis, and digastric muscles is not a secondary effect.
The Distances (mm) Between the Origin and Insertion of Masseter, Digastric, and Temporalis Muscles Analyzed by µCT
| Control | Clenbuterol | Significance | |
|---|---|---|---|
| Masseter | |||
| 1 | 9.87 ± 0.67 | 9.47 ± 0.74 | NS |
| 2 | 19.90 ± 1.22 | 19.35 ± 0.39 | NS |
| Temporalis | |||
| 3 | 10.10 ± 0.63 | 10.49 ± 0.50 | NS |
| 4 | 11.12 ± 0.66 | 12.07 ± 0.70 | NS |
| 5 | 14.20 ± 1.09 | 15.44 ± 0.82 | NS |
| Digastric | |||
| 6 | 13.52 ± 1.22 | 13.18 ± 0.48 | NS |
| 7 | 6.62 ± 1.05 | 7.05 ± 0.60 | NS |
The distance was expressed as the mean ± standard deviation for five rats.
DISCUSSION
In the present study, the hypertrophic rate associated with clenbuterol in the craniofacial muscles, including the masseter, temporalis, and digastric muscles, ranged from 36 to 56%, whereas those of the tibialis anterior, diaphragm, and heart ranged from 9 to 33%. Comparative data from previous studies of murine limb, trunk, and heart muscles are presented in tabular form in Table 2 [25]. The maximum value of the hypertrophic rate in the previous studies is 27%, which was induced in the soleus following 27 days of oral administration of 30 μg/ml of clenbuterol; the hypertrophic rates ranges from 0 to 27%. Since the dose, duration, and method of administration of clenbuterol varied among these studies, they are very difficult to compare with the present study. However, the hypertrophic rates shown in Table 2 are less than those of the craniofacial muscles in the present study.
Reported Hypertrophic Rate of Murine Limb, Trunk, and Heart Muscles Induced by Clenbuterol
| Dose (mg/kg/day) | Duration (days) | Method | Hypertrophic rate (%) | Heart | No. of Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sol | Gas | PI | EDL | TA | DP | |||||
| 0.01 | 14 | M.P. | 12 | -- | 18 | -- | 11 | -- | 12 | [25] |
| 0.25 | 7 | S.C. | 18 | 22 | 22 | -- | -- | -- | 0 | [26] |
| 0.25 | 56 | S.C. | 0 | -- | -- | 20 | -- | -- | -- | [6] |
| 1.0 | 14 | S.C. | 14 | 17 | 17 | 19 | 15 | -- | 10 | [27] |
| 1.0 | 21 | S.C. | -- | 5 | -- | -- | -- | -- | -- | [28] |
| 1.0 | 42 | S.C. | 17 | -- | -- | -- | -- | -- | -- | [29] |
| 2.0 | 16 | S.C. | -- | 13 | -- | -- | -- | -- | 0 | [2] |
| 2.0 | 14 | S.C. | 6 | 18 | 15 | -- | -- | -- | -- | [30] |
| 2.0 | 14 | S.C. | -- | 19 | -- | -- | -- | -- | -- | [3] |
| 2.0 | 105 | Oral | 17 | -- | -- | 0 | -- | -- | -- | [31] |
| 3.0 | 9 | M.P. | -- | 12 | -- | -- | 11 | -- | -- | [14] |
| # | 14 | Oral | 8 | 24 | -- | 19 | -- | -- | -- | [4] |
| # | 28 | Oral | 27 | -- | -- | -- | -- | -- | -- | [5] |
| 4.0 | 21 | Oral | 0 | -- | -- | -- | 33 | 17 | 9 | * |
The percentage of the wet weight of the soleus (Sol), plantaris (PI), extensor digitorum longus (EDL), tibialis anterior (TA), diaphragm (DP), and heart are expressed relative to the control. Dashes (--) indicate that the muscle mass was not measured. S.C., M.P., and Oral denote subcutaneous injection, continuous injection by subcutaneously implanted osmotic mini-pump, and oral administration, respectively.
* Data from the present study is included for comparison.
# In these two studies, 30 μg/ml of clenbuterol was orally administered to animals via their drinking water and the exact dose of clenbuterol was not determined.
Muscle satellite cells are activated and induce adaptive changes of skeletal muscle such as hypertrophy, the alteration of fiber type, and regeneration by external stimuli such as mechanical loading, unloading, denervation, and injury through growth factors such as IGFs, myostatin and IL-6 [16,21-23,32,33]. We have reported that the pool size of satellite cells in the masseter muscle of a muscle dystrophy model mouse (mdx) is greater than those of other muscles such as the gastrocnemius, soleus, and diaphragm [24]. It also has been also reported that clenbuterol stimulates the activation, proliferation and differentiation of satellite cells [34-36] and, in the mouse disrupting β2 adrenergic receptor gene, clenbuterol is not able to induce the hypertrophy of skeletal muscles [14]. These reports suggest a direct relationship among the satellite cells and hypertrophy of skeletal muscle induced by clenbuterol. In the present study, the clenbuterol-induced hypertrophic rates of craniofacial muscles were greater than those of the tibialis anterior, soleus, and diaphragm muscles, and greater than those seen in previous studies. This result supports our hypothesis that the hypertrophic effect of clenbuterol on the craniofacial muscles containing the masseter muscle is greater than on other muscles.
We found no significant difference induced by clenbuterol in the weight of the tongue between the control and clenbuterol groups. Although the tongue muscles constitute a subset of the craniofacial muscles, the developmental origins of tongue muscles involves the hypaxial somites 2 ~5, and not the somitomeres, which are involved in the developmental origin of the craniofacial muscles [37-39]. Further, the program governing tongue myogenesis is similar to that for limb myogenesis and distinct from that for craniofacial myogenesis [40]. These differences may be responsible for the observed differences in the hypertrophic effect on tongue muscles by clenbuterol relative to the other craniofacial muscles such as the masseter, digastric, and temporalis.
ACKNOWLEDGEMENTS
This study was supported in part by grants-in-aid for funding scientific research (No. 19592266 to C.I. and No. 20592190 to A.Y.), for funding the Bio-ventures and High-Technology Research Center from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, by the Science Research Promotion Fund from the Promotion and Mutual Aid Corporation for Private Schools of Japan, and by grant-in-aid for funding scientific research form the Society for Tsurumi University School of Dental Medicine.


