All published articles of this journal are available on ScienceDirect.
Treatment of Bisphosphonate-related Osteonecrosis of Jaw (BRONJ) in Rabbit Model: A Proof-of-concept Animal Study Comparing Angiogenesis Factor Versus Autologous Bone Marrow-derived Osteoblasts (ABMDO)
Abstract
Objective
We created Bisphosphonate-related Osteonecrosis of Jaw (BRONJ) in rabbits and treated them with an angiogenesis factor or autologous bone marrow derived osteoblasts (ABMDO) to assess the efficacy of the treatment by Micro-computerized Tomography (M-CT) and histopathology.
Materials and Methods
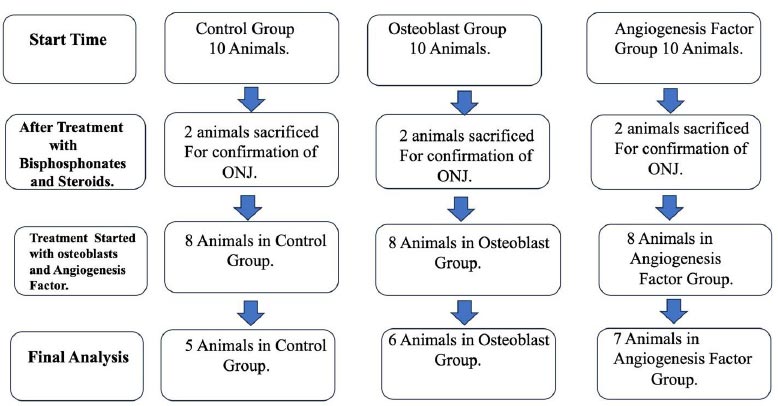
Thirty female New Zealand rabbits were procured and were divided into three groups of 10 animals each. The number of animals to achieve statistical significance was based on the reported studies. Group I was control group (C), Group II was Osteoblast group (O), and Group III was angiogenesis group (P). In all Groups, BRONJ was produced. At 8 weeks of tooth extraction, BRONJ was confirmed histologically and radiologically in two rabbits from each group of animals. Group I received 0.5 of normal saline, Group II received a single dose of 5 million osteoblasts suspended in 0.5 ml, and Group III received 5 mg of angiogenesis factor thrice weekly for three weeks. The healing of BRONJ was assessed using M-CT and histopathology.
Results
In O and P groups, the extraction sockets healed and closed with normal-looking tissue, whereas in the C (control) group, suppuration with an area of necrosis was observed. Micro-CT analysis of socket revealed an exaggeration on non-mineralized soft tissue volume in the C group, whereas most of the bone promotion parameters were improved in the O and P groups with statistical difference (P<0.001) for the parameters bone volume, bone surface area, trabecular number and trabecular thickness. Histologically, the element of healing was represented by reactive bone formation and fibrosis, which were more prominent in groups O and P as compared to the control group.
Conclusion
Our study shows that ABMDO and angiogenesis factor have a robust potential to heal BRONJ.
Clinical Relevance
The study shows angiogenesis factor and osteoblasts heals BRONJ and warrant sincere human trials to tackle this unrelenting complication of bisphosphonates use.
1. INTRODUCTION
Bisphosphonates prevent the loss of bone density and are used to treat osteoporosis and similar diseases. Before osteoporosis, bisphosphonates were used in cancer therapy. Bisphosphonates are used in cancer therapy to reduce bone pain and the risk of fractures [1-4]. It was reported that in some cancers, they also improve patient survival [5, 6]. At present, bisphosphonates are the choice of drug used in the treatment of post-menopausal osteoporosis [7-9].
Bisphosphonates-related osteonecrosis of the jaw (BRONJ) is one of the well-described complications of bisphosphonate therapy and affects the jaws, either mandible or maxilla and in 2003, BRONJ became a pathological continuant entity [10]. Many theories have been put forward for the cause of BRONJ. One theory put forward the impaired functionality of macrophages as the dominant factor in the development of BRONJ, and the hypovascularity of the mandible itself has been suggested to be an important factor [11, 12]. Recently, apart from other causes, impaired angiogenesis has been blamed at the site of BRONJ [13, 14].
Further, various treatments were tried, but the consequences of the BRONJ and cure appeared afar. Since no treatment was efficacious, a multitude of therapies were attempted [15-17]. Nonetheless, an effective treatment for BRONJ still remains elusive and further options need to be investigated [18]. A novel model of BRONJ in rats was introduced, which allows for more effective treatment of BRONJ [19, 20]. A recent study in rats showed that BRONJ can be treated successfully with angiogenesis factor and by ABMDO [21]. In this study, we created BRONJ in rabbits using intravenous bisphosphonates and extraction of the 1st premolar. We treated it with ABMDO and angiogenesis factor and confirmed the effectiveness as previously indicated in smaller animals.
2. MATERIALS AND METHODS
The study was performed based on the ARRIVE guidelines and approved by the Institutional Review Board and Animal Care Committee of the Imam Abdul Rahman Bin Faisal University, Dammam, Saudi Arabia, vide #2021-01-464.
Thirty female New Zealand rabbits were procured from in-house breeding at the Animal House of the Institute for Research and Medical Consultations (IRMC), Imam Abdul Rahman Bin Faisal University, Dammam. The number of animals chosen to achieve statistical significance was based on the reported studies. Group I was the control group (C), Group II was the Osteoblast group (O), and Group III was the angiogenesis group (P). The number of animals was decided on the basis of achieving accepted statistical significance [21, 22]. In all Groups, BRONJ was created by intravenous zoledronate 800 μg/kg and dexamethasone 10 mg/kg body weight once a week for 8 weeks and extraction of the mandibular first premolars after 6th week under general anesthesia. After 8 weeks of tooth extraction, BRONJ was confirmed histologically and radiologically in two rabbits from each group. All animals received their respective doses at the site of the molar extraction. Group I (C; n=8) received 0.5 of normal saline, Group II (O; n=8) received 5 million osteoblasts suspended in 0.5 ml, and Group III (P; n=8) received 5 mg of angiogenesis factor dissolved in 0.5 ml of normal saline thrice weekly for three weeks.
After four weeks, animals were euthanized, and each mandible was harvested en bloc and fixated in 10% formalin. The healing of BRONJ was assessed using a high-resolution micro-CT Skyscan 1172 machine (Bruker Corporation 40 Manning Rd, Billerica, MA 01821, United States). The parameters used were as follows: source voltage=100kV, current=100uA, image pixel size=13.73 um, filter=Al, and rotation=360. Software used were NRecon (version 1.6.4.8) for image reconstruction, CTAnalyser (version 1.11.10.0) for 3D image analysis, and Dataviewer software for 2D/3D Micro-CT sliced visualization (all from Bruker Corporation 40 Manning Rd, Billerica, MA 01821, United States). After micro-CT, the specimens were decalcified and embedded in paraffin for qualitative histological evaluation using hematoxylin and eosin staining. In addition, selected samples were processed of immunostaining targeting blood vessels proliferation marker CD31 (Abcam, Waltham, MA, USA).
2.1. Statistical Analysis
Statistical analysis was conducted using IBM SPSS® software (Version 29). For the total socket area as a region-of-interest (ROI), one-way ANOVA followed by Tukey's post-hoc test was used, considering the interventions as the only variable. When dividing the socket into 3 ROIs, two-way ANOVA was conducted using the intervention (BRONJ; BRONJ + therapy P (Angiogenesis Factor); BRONJ + therapy Osteoblasts) and the location of the ROI (Top; Middle; Bottom) as the two study variables. In conjunction with the two-way ANOVA, Bonferroni's test was used, which adjusts for the multiple comparisons. Data are presented in column graphs showing the mean and the standard error of the mean. Statistically significant differences (P values <0.05 and <0.001) are presented on the graphs.
3. RESULTS
Death occurred in the control group and group II (O group) after the treatment was instituted. The relatively higher mortality in the C group can be explained by the prolonged inflammatory processes, which might have extended beyond the local nature to a systemic condition. In the same context, the possibly reduced inflammation and enhanced regeneration in the O and P groups might have had a better effect in mitigating the development of systemic inflammation. BRONJ was confirmed histologically and radiologically in 2 animals in all the groups. In the O and P groups, there was the closure of the overlying soft tissue, whereas, in the C=Control group, there was suppuration and discharge with an area of mortification. Figs. (1 and 2) shows the total number of animals analyzed.
Generally, the extraction sockets appeared to be healed in the O and P groups, with a normal appearance of the mucosa covering the extraction site. In contrast, extraction sockets in the C group exhibited various degrees of necrotic tissue and suppuration.
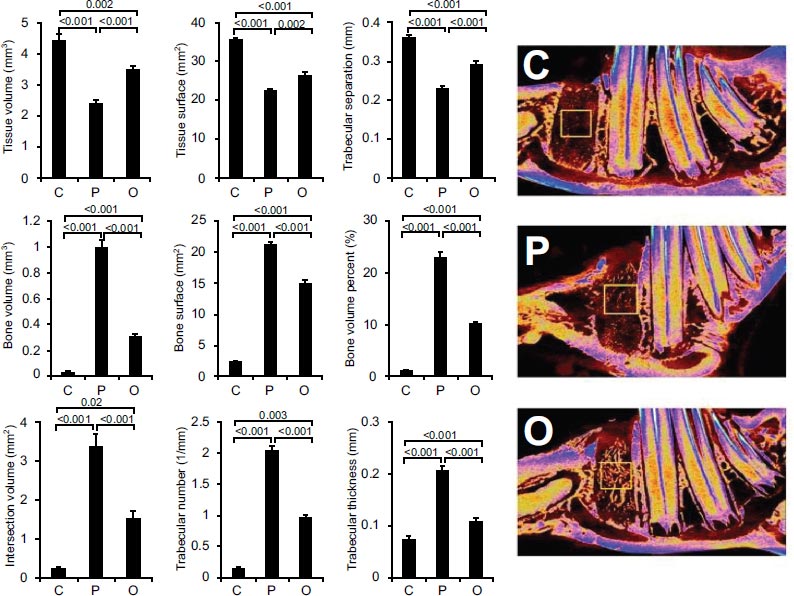
Figs. (3-5) show the micro-CT evaluation of the extraction sockets in the three groups at three different ROIs (Top, middle and bottom, respectively).
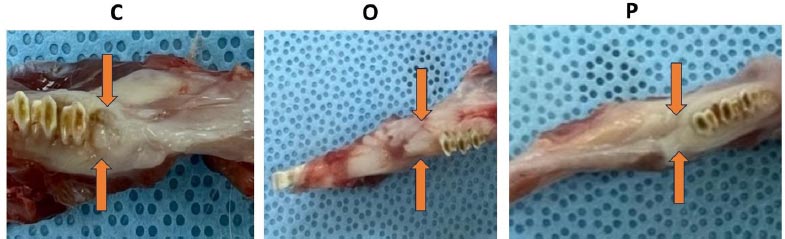
Gross pictures of control group=C, Osteobast=O and Angiogenesis factor=P.
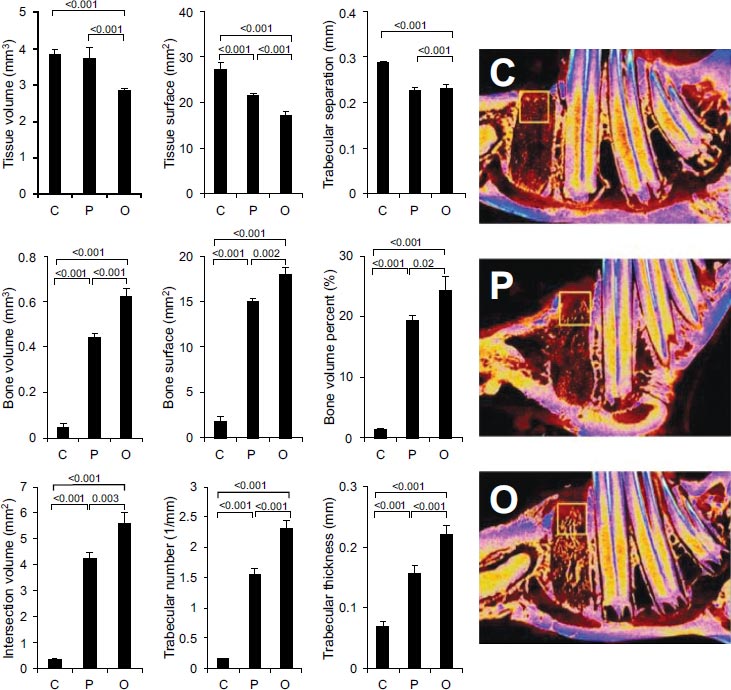
In all ROIs, the control group (C) always showed an exaggerated soft tissue parameter, indicating an ongoing process of necrosis, as well as an increased trabecular separation, indicating higher bone resorption activity. In contrast, the bone promotion parameters, including bone volume and surface area, as well as the trabecular number and thickness, were generally higher in the angiogenesis (P) and the osteoblast (O) groups. In the top ROI, the comparison between the O and P groups revealed that the O group was significantly better than the P group for bone surface area (p=0.002), bone volume, trabecular number, and trabecular thickness (p<0.001). In the mid ROI, the P group was significant in all parameters (p<0.001) when compared to the O group and C groups (Fig. 4).
In the 3rd ROI, the effect was comparable between the O and P groups and better than the C group at p<0.001 (Fig. 5).
Histologically, the element of healing was represented by reactive bone formation and fibrosis, which were more prominent in groups O and P as compared to the control group. Both the groups had comparable extent of reactive bone formation (Fig. 6). However, fibrosis was slightly more prominent in group O. Skeletal vasculature played a significant role in the process of bone development, regeneration and remodeling.
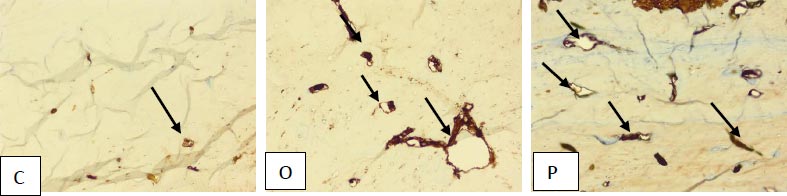
Reactive vascular proliferation was also identified which was more pronounced in group O and P as compared to group C. The CD-31 immunostaining, a vascular proliferation marker, was conducted to assess the extent of proliferating vessels (Fig. 7). The varying extent of acute and chronic inflammation was seen in all groups. There was no evident cartilaginous proliferation in any of the groups.

Figures C2, O2 and P2, H &E x400 show reactive bone formation (single arrow) with background stroma (*) showing edema in C2 (control), marked fibrosis in O2 (ABDMO) and mild fibrosis in P2 (Angiogenesis factor).
Figures C3, O3 and P3, H &E x400, highlight the extent of vascular proliferation (*) with moderate proliferation seen in O3 (ABDMO) and marked seen in P3 (Angiogenesis factor). A single arrow shows reactive bone formation.
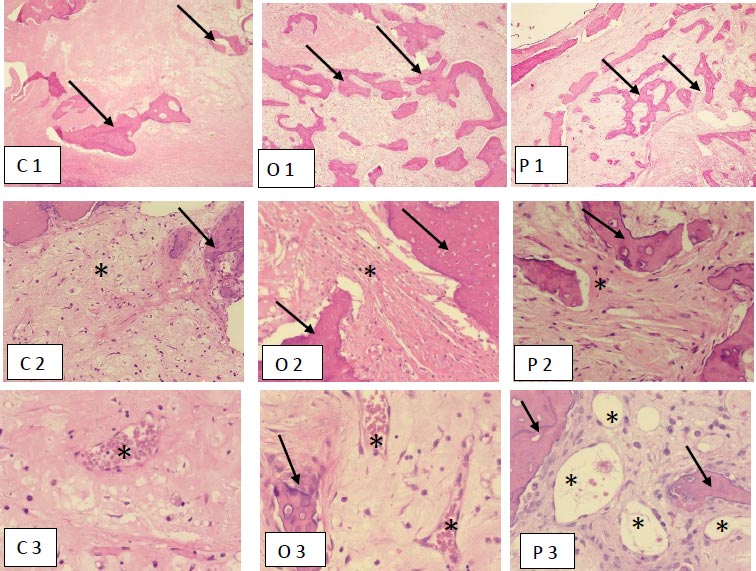
Figures C2, O2 and P2, H &E x400 show reactive bone formation (single arrow) with background stroma (*) showing edema in C2 (control), marked fibrosis in O2 (ABDMO) and mild fibrosis in P2 (Angiogenesis factor).
Figures C3, O3 and P3, H &E x400, highlight the extent of vascular proliferation (*) with moderate proliferation seen in O3 (ABDMO) and marked seen in P3 (Angiogenesis factor). A single arrow shows reactive bone formation.
4. DISCUSSION
Our study shows that BRONJ can be successfully treated with ABMDO and angiogenesis factors. This study observed that the effect was different in three different ROIs: at the top of the socket, the middle, and the bottom of the socket. In all three ROIs, the healing was superior in the treatment groups in comparison with the control group. This study correlates to the earlier report in experimental rats, where a similar methodology was used [23].
The incidence of BRONJ varied from 0.01% to 9.9% depending on risk factors as those treated for malignant conditions [24, 25]. Fantasia (2015) [26] reported that inhibition of angiogenesis likely has either a primary or secondary role in the development of ONJ. Angiogenesis has long been earmarked for anticancer therapies, which could reduce the growth of tumors [27]. The increased risk of anti-angiogenesis is due to the importance of normal vessel growth in wound healing. Added to this, BRONJ is routinely reported in cancer patients using anti-angiogenesis therapy [28, 29]. Our study perceived that the effect of the angiogenesis factor in the healing of BRONJ was excellent in comparison to the control group of animals.
Recently, studies have demonstrated that stem cell therapy has great promise for clinical applications and robust therapeutic effects in bone repair [30, 31]. Stem cell therapy has reported exceedingly good results in the treatment of osteonecrosis of the head of the femur [32-34]. This led researchers to use mesenchymal stem cells (MSCs) as a therapeutic modality for tissue regeneration. Moreover, bone marrow-derived MSCs can differentiate into osteogenic cells and form new bone. MSCs have been proven to be effective in MRONJ in animal models [35]. Nonetheless, the relatively complex isolation and characterization of MSCs, in addition to their potential differentiation to other cell types than the osteogenic lineage, may pose some disadvantages. Here, we used ABMDO instead of MSCs and found it to be very effective in the mitigation of BRONJ. In the management of BRONJ, a state of clinical equipoise exists, but recently, in a systematic review and international consensus, Khan et al. (2015) [36] concluded that in BRONJ, bone marrow stem cell intralesional transplantation is one of the modalities of treatment.
CONCLUSION
First of all, our study had limitations in the disparity of the number of animals in each group. There were only 5 animals in the control group compared to 8 in other groups. Secondly, we were unable to explain the mechanism of the positive effect of the two treatment modalities, as well as the site-specific variations of these effects in the three ROIs. The strength of our study is that we showed, by micro-CT (which evaluates the 3D structure) and histopathology, that the study groups had significantly more reactive bone formation and healing of BRONJ. Nonetheless, we believe that the results of our study warrant phase I human trials to test the efficacy and safety so that a cure for this severe debilitating complication can be found.
AUTHORS’ CONTRIBUTION
1. Mir Sadat-Ali contributed to the concept, follow-up of animals, injection of the osteoblasts and angiogenesis factor, review of literature, writing and completion of the manuscript.
2. Omar M Omar contributed to the extraction of the teeth, follow-up at the animal house, and review of the manuscript.
3. Khalid Almas contributed to the extraction of teeth, follow-up of animals, and correction of the final manuscript.
4. Ayesha Ahmed contributed to the review of literature, histopathology studies and reporting, and the final review of the manuscript.
LIST OF ABBREVIATIONS
| ABMDO | = Autologous Bone Marrow-derived Osteoblasts |
| M-CT | = Micro-computerized Tomography |
| C | = Control Group |
| O | = Group II Osteoblast Group |
| P | = Group III Angiogenesis Group |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This animal study was approved by the Institutional Review Board and Animal Care Committee of Imam Abdul Rahman Bin Faisal University, Dammam, Saudi Arabia, vide #2021-01-464.
HUMAN AND ANIMAL RIGHTS
No humans were used in this study. All the procedures involving animals were performed according to the US Public Health Service's “Policy on Human Care and Use of Laboratory Animals,” and “Guide for the Care and Use of Laboratory animals” and in accordance with ARRIVE guidelines.
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
FUNDING
The study was funded by the Deanship of Scientific Research, Imam AbdulRahman Bin Faisal University, Dammam, Saudi Arabia; Vide # No. 2021-206.


