Association of TAS1R2 (rs35874116 or rs9701796) Gene Polymorphism with Dental Caries: A Systematic Review and Meta-analysis
Abstract
Background
Gene variations affecting taste preference and glucose consumption have recently been linked to dental caries.
Aims
Possible associations between dental caries and the TAS1R2 gene polymorphisms rs35874116 and rs9701796 have been investigated, but conflicting results have been found. Therefore, a meta-analysis was conducted to find comprehensive and more specific results in this direction.
Methods
The work started by searching English articles until April 2023 from the reliable databanks PubMed, Embase, Scopus and Web of Science. Then, relevant articles were carefully chosen in various steps based on defined selection criteria and assessed by comprehensive meta-analysis v.2.0 software.
Results
Based on six selected articles, the meta-analyses showed a significant association between dental caries risk and the TAS1R2 gene polymorphism rs9701796 (p<0.05), with the GG genotype in rs9701796 increasing the risk. No significant relation was found for rs35874116.
Conclusion
This meta-analysis concluded that the rs9701796 polymorphism increases the risk of dental caries. Studies with larger sample sizes can clarify the relationship further.
1. INTRODUCTION
Dental decay is one of the most common diseases that people deal with [1]. Bacteria colonized in dental plaque ferment carbohydrates to make acid, which dissolves the mineral matrix of enamel and causes tooth decay. The composition of oral microflora, oral hygiene, diet, and factors related to the host will have a serious role in tooth decay. Environmental and social factors, such as fluoride use and preventive care, also affect the caries process [2-4].
The complex interaction of several different risk factors, including environmental factors and individual genetic predisposition, represents the true multifactorial etiology of this disease [5, 6]. Various studies conducted on twins showed that the genetic background of individuals determines 40-60% of caries susceptibility [7-11]. Factors that are genetically involved in tooth decay include tooth morphology, tooth enamel structure, saliva quality and flow, nutrition, and taste preferences [12-17]. It has also been proven that one of the important factors of caries is nutritional habits, including repeated consump- tion of metabolizable sugar by oral microorganisms [18-20].
The desire to consume sugary substances, which happens due to the different perceptions of sweet taste by people, is a predisposing factor for tooth decay [21, 22]. Fungal papilla number and phenotypic variation in taste receptors from gene coding polymorphisms, such as taste receptor type 1, member 2 and 3 (TAS1R2 and TAS1R3) for sweet taste receptors and taste receptor type 2 (TAS2R38) for bitter taste, determine the acceptance of taste by people [23-26].
Sugar intake and sweet taste preference are associated with genetic polymorphisms in human receptors [21, 27-29]. The association between sugar consumption and alleles of the sweet taste receptor (TAS1R2) has been established in humans [30]. The sweet taste receptors are encoded by the TAS1R2 and TAS1R3 gene receptor proteins placed on chromosome one [31-35]. It should be noted that the TAS1R gene family is involved in sweet taste sensitivity [33, 36]. The role of the candidate gene TAS1R2 in identifying sweet taste in the palate and tongue is undeniable. Also, it affects food consumption, which can be due to the high tissue diversity in the location of this receptor [37]. TAS1R2 has two common polymorphisms. The minor allele frequency of its polymorphisms is above ten percent. One of the polymorphisms named Ile191Val (rs35874116), is positioned in one of the binding sites of this protein to its corresponding ligand [33], and a considerable relationship between TAS1R2 and dental caries has been detected in several studies [17, 38]. The next polymorphism is rs9701796, Wendell et al. [34] showing that TAS1R2 rs9701796 is significantly associated with caries.
To investigate the association between the TAS1R2 gene and sweet consumption as well as sugar-related caries, research works focused on the association between polymorphisms of rs9701796 and rs35874116 in the TAS1R2 gene with consuming sweets and tooth decay in recent years. Although several studies have been conducted on this type of genetic polymorphism, no meta-analysis has been implemented to confirm or reject the relationship of this gene in causing caries. Previous studies have conflicting results. In the current article, we attempted a meta-analysis using the articles of the last few years to find the relationship between the risks of caries in people with the TAS1R2 gene. Identifying the molecular aspect of caries-causing factors leads to the identification of innovations in the field of early detection of people at caries risk and the implementation of low-cost primary preventive care, which ultimately improves oral health.
2. MATERIALS AND METHODS
2.1. Methodology
The registration of the review protocol was completed on the PROSPERO database with registration ID CRD42023 448165. A comprehensive process was applied for the current review in which the analysis and eligibility criteria were specified based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. In this study, Population, Intervention, Comparison, Outcomes, and Study design (PICOS) criteria were as below:
2.1.3. Comparisons
The association of TAS1R2 gene polymorphism (rs35874116 or rs9701796) in individuals with tooth decay compared with individuals without tooth decay.
2.1.4. Outcome
Papers assessing the relationship between TAS1R2 gene polymorphisms (rs35874116 or rs9701796) with dental caries in different genetic models.
2.1.5. Study Design
Observational studies using valid processes.
In this research, our research question was whether the TAS1R2 gene polymorphism (rs35874116 or rs9701796) in individuals with tooth decay is equal to that in caries-free people.
A library search of PubMed, Embase, Web of Science and Scopus databanks among the published articles up to April 2023 was performed. Related references were searched in the selected items. We used the MESH (Medical Subject Heading) and free terms to receive evidence. The nominated keywords in this study were: “dental caries” OR ” DMFT ” OR ” dmft ” AND “single nucleotide polymorphism” OR ” genetic susceptibility ” OR ” mutation ” OR “polymorphism” OR “SNP” and ” TAS1R2” OR “rs35874116” OR “taste receptor” or “rs9701796”.
2.2. Selection of Study Items
After the required documents were obtained from the database records, they were evaluated by two experts in three stages. At first, two reviewers independently screened the abstracts and titles, considering the appropriate criteria. Any case of disagreement was discussed and resolved with the third reviewer. Then, the full text of the selected articles was studied. The Newcastle-Ottawa scale [39] has been adopted to grade the research quality, and the highest score for each article is 9. If a study has a score equal to or greater than 7, it demonstrates a high quality of research. The main features of the studies were summarized in a simple table. Further details, including author name, country, publication year, control source, sample size (case/control), method of genotyping and the location of the polymorphism, are included in the sheet.
Inclusion criteria include cross-sectional and case-control studies, studies that observe the TAS1R2 gene polymorphism (rs35874116 or rs9701796) in both individuals with dental caries and the control group, with no other disease in both cases and controls. Other exclusion criteria include the following: animal studies, case reports, reviews, replicated studies, letters to the editor, studies that evaluated other SNPs of the TAS1R2 gene polymorphisms, and studies with insufficient data for analysis.
2.3. Statistical Analysis
We used a 95% confidence interval (CI) and odds ratio here to compare control and case subjects in terms of gene polymorphism. The total odds ratio (OR) of all genetic models, including allelic, heterozygous, homo- zygous, recessive and dominant models, was calculated. Adjusted ORs were extracted from the original included studies, absolute numbers being used to estimate ORs and 95% CIs when no effect size was reported. The random-effects model was used to estimate the pooled effect size with 95% CIs. To indicate heterogeneity between studies, we used the I2 statistic and Cochran's test in this analysis. For the I2 coefficient less than 50% and P greater than 0.1, there was no significant heterogeneity between studies. Then, comprehensive meta-analysis software version 2.0 was applied for meta-analysis, and the P<0.05 criterion was used to report the statistical significance of the data. All analysis was carried out in STATA version 14.0 (StataCorp, College Station, TX, USA).
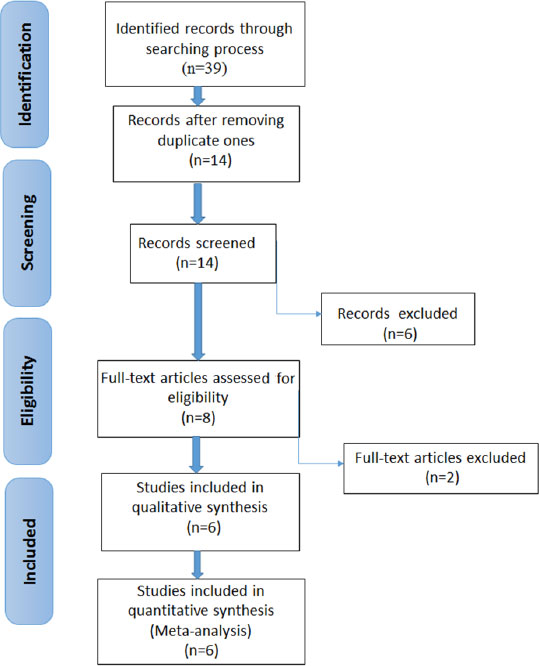
3. RESULTS
3.1. Selection of Studies
Table 1 shows the information about the genes included in this review. Fig. (1) shows the search strategy and the number of studies included in the meta-analysis. In the initial stage of the research, 39 articles were found. Then, reduplicative and unfitting articles were put aside, and six articles [33, 36, 38, 40-42] were selected for evaluation; six of these evaluated the rs35874116 gene polymorphism and three examined the rs9701796 gene polymorphism. The features of the selected articles are summarized in Table 2.
| Gene | TAS1R2 |
|---|---|
| dbSNP ID | rs35874116 |
| rs9701796 | |
| Location (chromosome, base position) |
1p36.13 |
| Exon count | 6 |
| Assembly | GRCh38.p14 |
| Alleles | T/C |
| C/G | |
| Minor allele | C |
| G | |
| Reference | (1) |
| Publication Year | Country | Case (M/F) | Control (n) | Age (year) | Genotyping Method | Location of Polymorphism | Major Findings |
|---|---|---|---|---|---|---|---|
| 2022 | China | 118(61/57) | 118 (55/63) | 5 | PCR | TAS1R2 (rs35874116) | The rs35874116 polymorphism may increase the consumption of sweets and the possibility of severe- early childhood caries (S-ECC) among Chinese children. They showed carriers of the TT genotype are at high risk of permanent teeth caries. |
| 2021 | Turkey | 205(91/114) | 51(21/30) | 18-45 | PCR | TAS1R2 (rs35874116 or rs9701796) | TAS1R2 gene polymorphism (rs35874116, rs9701796) cannot be related to dental caries in Turkish people. |
| 2020 | China | 382(202/180) | 673(413/260) | 13-14 | PCR | TAS1R2 (rs35874116 or rs9701796) | No selected SNPs (e.g., TAS1R2 rs35874116, rs9701796) showed significant association with tooth decay under either dominant or additive models. |
| 2015 | Turkey | 127(56/71) | 55(26/29) | 7-12 | PCR | TAS1R2 (rs35874116 or rs9701796) | TAS1R2 gene polymorphism (rs35874116, rs9701796) cannot be related to dental caries in Turkish people. |
| 2015 | Czech | 482(242/240) | 155(82/73) | 11-13 | PCR PCR |
TAS1R2 (rs35874116) | A polymorphism in TAS1R2 (rs35874116) was related to tooth decay and it concluded that dental caries affect people with the Val allele for the TAS1R2 polymorphism more than people with the Ile allele. |
| Criteria | Studies | ||||||
|---|---|---|---|---|---|---|---|
| Chisini et al. | Liang et al. |
Yilmaz et al. |
Wang et al. |
Haznedaroğlu et al. |
Izakovicova Holla et al. |
||
| Selection | Case definition adequate | + | + | + | + | + | + |
| Representativeness of the cases | + | + | + | + | + | + | |
| Selection of controls | + | + | - | - | - | + | |
| Definition of controls | + | + | + | + | + | + | |
| Comparability | Main factor* | + | - | - | - | + | + |
| Additional factory | + | - | + | + | - | + | |
| Outcome | Ascertainment of exposure | + | + | + | + | + | + |
| Same method of ascertainment for cases and controls | + | + | + | + | + | + | |
| Nonresponse rate | + | + | + | + | + | + | |
| Total score | 9 | 7 | 7 | 7 | 7 | 9 | |
ySex was matched between two groups.
zGood quality (score: >7) and fair quality (score: 5-7).
3.2. Quality Evaluation
The average quality of the articles included in our meta-analysis was 7.6, which is shown in Table 3. All articles were highly rated.
3.3. Meta-analysis Results
Six articles have been used in our meta-analysis. These papers assessed the relationship between TAS1R2 gene polymorphisms (rs35874116 or rs9701796) with dental caries in different genetic models. Using the random effects model, no significant heterogeneity was found in all the forest plots (I2 < 50, P-value > 0.1), except the comparison of C vs. T.
According to the results of the meta-analysis, the association between TAS1R2 gene polymorphism (rs35874116) and tooth decay is summarized in the forest plot (Fig. 2). This meta-analysis included 1693 patients with tooth decay and 1285 people without tooth decay. The results suggested that the CC genotype might be associated with tooth decay; however, no significant association was found.
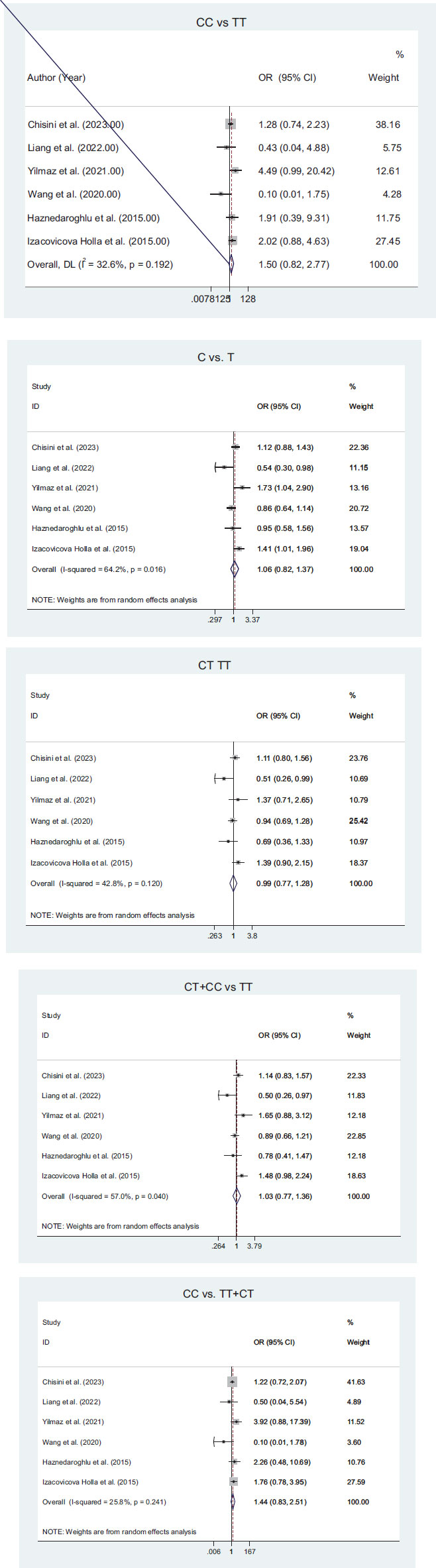
Fig. (3) shows a forest plot of the association between TAS1R2 gene polymorphism (rs9701796) and tooth decay. The analysis included three studies. In this meta-analysis, 659 caries patients and 779 caries-free patients were included. A significant relationship was observed between the polymorphism of this gene and the risk of caries for the GG genotype. For other genotypes, no statistically significant association were reported.

4. DISCUSSION
Dental caries is affected by various factors, such as genetic predisposition, oral hygiene habits, taste preferences and diet [7, 8, 34, 43, 44]. Recently, various genome-wide studies have been performed to recognize candidate regions and caries-related genes [45-49]. Studying the molecular aspect of dental caries makes it possible to take an innovative and objective approach to its prevention and management [40]. One of the causes of tooth decay is the genetic variation that determines the inclination for sugar consumption and the sweet taste sensation. Perception of sugary taste is mediated by a G protein coded in the TAS1R (33,34). Where TAS1R1 and TAS1R2 (taste receptor proteins) are mainly expressed in mushroom-shaped papillae on the borders and tip of the tongue and palatal taste receptor cells [49].
The current investigation presents evidence for the genetic variation of rs9701796 in TAS1R2 to be related to dental caries. Although for genetic variation of rs35874116, we found no significant association, the values differed between those with dental caries and healthy controls. It can be said that the polymorphism of rs35874116 is located in the first predicted huge extracellular domain of the T1R2 receptor, which contains a binding site for dipeptide sweeteners and carbohydrate ligands [50, 51], and as a result, it affects sugar-dependent disease, such as tooth decay [38].
In this systematic review, we assessed the impact of TAS1R2 polymorphisms (rs35874116 and rs9701796) on the possibility of caries formation. We included six studies in our meta-analysis. We concluded that the CC genotype at rs35874116 and the GG genotype at rs9701796 are associated with higher caries experience.
First, we review studies that investigated the relationship between caries and TAS1R2 (rs35874116 and rs9701796) polymorphisms. Yilmaz et al. concluded that TAS1R2 gene polymorphism (rs35874116, rs9701796) cannot be related to dental caries in Turkish people [41], a conclusion that was almost identical to Haznedaroğlu et al.'s study [36]. Izakovicova Holla et al. [40] reported that a polymorphism in TAS1R2 (rs35874116) was related to tooth decay. They concluded that dental caries affect people with the Val allele for the TAS1R2 polymorphism more than people with the Ile allele. Wang et al. [42] reported that no selected SNPs (e.g., TAS1R2 rs35874116, rs9701796) showed significant association with tooth decay under either dominant or additive models. Liang et al. [38] indicated that the rs35874116 polymorphism may increase the consumption of sweets and the possibility of severe- early childhood caries (S-ECC) among Chinese children. They showed carriers of the TT genotype are at high risk of permanent teeth caries. Kilic et al. [52] reported that there is no statistically considerable difference between the case and control groups in the DMFT/dmft index according to TAS1R2 rs9701796 SNP,, but homozygous CC genotype carriers had high DMFT/dmft scores.
The studies conducted have different results, and this difference is likely to be due to genetic differences in different populations, interaction of genes and environment, type of study design, diet and inheritance mode [38].
The results of these studies should be interpreted carefully because most of these studies have limitations, as mentioned below. First, most of these studies examined only one SNP locus in TAS1R2 and did not examine other SNPs that may be associated with tooth decay [38]. Second, information on dietary habits such as sugar intake frequency, saliva flow, and salivary bacteria levels was not examined in many of these studies [40]. Third, the design of case-control studies is such that there is a possibility of false positive results, particularly when controls and cases are from various populations [40]. But this was not the case in Liang et al.'s study [38], which was a cohort. Fourth, in most studies, participants were recruited from people who came for check-ups so that they might have better oral conditions than the general population [40]. Another shortcoming of the studies was the limited number of samples, but the sample size in the study by Izakovicova Holla et al. [40] and Liang et al. [38] was relatively large. Liang et al. evaluated data on gingival index, plaque index and differential index, which indicated good oral status in their group [38].
Therefore, the analysis of this paper shows the significance of insight into the role of taste preferences on tooth decay. The result of this study helps to identify high-risk people before caries formation and take targeted preventive measures for them [50].
Finally, individuals with a taste gene polymorphism predisposing to dental caries should be advised to follow a diet, increase oral health care, and control caloric intake [52]. However, to confirm the findings of this study and investigate other polymorphisms related to taste receptors, further investigation with larger sample sizes could improve our findings. The effects of socioeconomic conditions, fluoride use, and self-care of teeth should also be checked. Thus, subsequent evaluations are suggested to develop these findings in various populations and investigate some others that may cause tooth decay. Work on understanding polymorphisms of the genes that can alter susceptibility to tooth decay enables clinicians to identify at-risk patients before developing frank lesions.
CONCLUSION
Meta-analysis of this study showed a significant association between TAS1R2 (rs9701796) gene polymorphism and tooth decay. However, no significant association was found for TAS1R2 (rs35874116). The results show that the GG genotype in rs9701796 increases the risk of dental caries. However, well-designed studies with larger sample sizes could confirm these results in the future. Studying the genetic etiology of caries at the molecular level increases awareness about the genes involved in caries. It predicts it before its formation so we can carry out genetic interventions for prevention.
AUTHORS’ CONTRIBUTIONS
All authors have read and agreed to the published version of the manuscript.
ABBREVIATIONS
| PICOS | = Population, Intervention, Comparison, Outcomes, and Study design |
| CI | = Confidence Interval |
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
SUPPLEMENTARY MATERIAL
PRISMA checklist is available as supplementary material on the publisher’s website along with the published article.
Supplementary material is available on the publisher’s website along with the published article.


