All published articles of this journal are available on ScienceDirect.
A Systematic Review of Antibiotic Use in Dental Implant Therapy: Efficacy, Guidelines, and Recommendations
Abstract
Objective:
The objective of this research is to respond to that specific question: Are antibiotics needed for the placement of dental implants?
Methods:
A systematic search was done, and the selected studies were pooled from MEDLINE/PubMed, and Cochrane Library databases up to March 2022. Articles in which the main objective was to evaluate the relationship between antibiotics and dental implants were selected. The first search was done with Medline and Cochrane Library.
Results:
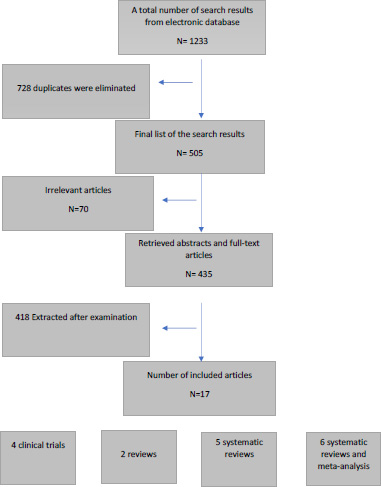
A total of 17 articles were finally included in the present review. Four clinical trials (CTs), two reviews, and eleven systematic reviews (SR); six of them with meta-analysis.
Conclusion:
Antibiotics may be used in dental implants to prevent or treat infections that may occur after implant surgery. The type and duration of antibiotic therapy depend on the patient’s medical history, the type of implant surgery being performed, and other factors. However, the use of antibiotics in dental implant surgery is debatable, and it is important to note that antibiotics should not be used routinely in dental implant surgery. Overuse of antibiotics can lead to antibiotic resistance which can make it harder to treat infections in the future. Thus, antibiotics should only be used when necessary and prescribed by a qualified healthcare professional.
1. BACKGROUND
One of the many causes of dental implant failure is the growth of bacteria surrounding the implants. Dental implants may develop bacteremia while being surgically implanted after the implant is placed before crown placement, or after the abutment and implant crown have been delivered. This could cause the area around the implant to lose soft or hard tissue (peri-implantitis). Using antibiotics as a preventative measure before surgery and after implant placement is intended to stop infections in the early stages of implant therapy. This review aims to provide information about the use of antibiotics in dental implants.
1.1. Introduction
Today’s dentists place a high priority on maintaining their patients’ oral health to its normal state in terms of appearance, functionality, comfort, cosmetics, and health. Ineffective oral function, poor esthetics, and structural imbalance are all consequences of tooth/teeth loss. In addition to the mouth’s masticatory and aesthetic requirements, maintaining occlusal function and optimum oral health depends on replacing the missing tooth or teeth. Since ancient times, many techniques have been utilized to replace missing teeth with artificial or natural counterparts, including removable partial dentures, fixed partial dentures, and complete dentures.
Dental implants are artificial tooth roots that are surgically placed into the jawbone to support a replacement tooth or bridge [1]. They are the most recent treatment option for individuals who have lost teeth due to injury, decay, or other reasons. While dental implants are phenomenally successful, they can be subject to bacterial infection, leading to implant failure, which can occur for a variety of causes. One example is the growth of bacteremia near implants. Antibiotics are often prescribed before and after dental implant surgery to prevent or treat any potential bacterial infections. However, the use of antibiotics in dental implant surgery is controversial and depends on various factors such as the patient’s medical history, the type of implant surgery, and the surgeon’s preference. In some cases, antibiotics may be unnecessary, as the risk of infection may be low. However, for patients with certain medical conditions such as a weakened immune system or a history of infections, antibiotics may be necessary to prevent complications. It is important to note that antibiotics should only be used as prescribed by a qualified dental professional. Overuse or misuse of antibiotics can lead to antibiotic resistance and other negative health consequences [2, 3].
2. MATERIALS AND METHODS
2.1. Search Strategy
Papers on local and/or systemic antibiotics and dental implants were searched electronically, in MEDLINE/PubMed, and Cochrane Library databases. To locate more relevant research to add to the review, reference lists from existing studies were also used as a search tool. The keywords used most frequently in searches were “antibiotic,” “antibiotic and dental implants,” “locally delivered antibiotics,”, and “systemic antibiotics”.
3. RESULTS
A total of 1233 records were identified from the electronic search, MEDLINE/PubMed, and Cochrane Library databases. In total, 728 duplicates were eliminated. Of the remaining articles that were potentially eligible, 70 citations were excluded, mostly because they did not meet the inclusion criteria. Seventeen articles were relevant and compliant with the inclusion criteria for the present review. Fig. (1) presents a flow diagram of the study selection process.
4. DISCUSSION
Ever since dental implants were used to replace missing teeth antibiotics have been used to prevent and treat implant failure. Antibiotic prophylaxis has been proven to effectively prevent surgical site infections and is a standard procedure in surgical practice. Several antibiotic administration strategies, such as antibiotic prophylaxis before surgery without post-surgical antibiotics and antibiotic treatment after surgery with or without antibiotic prophylaxis, have been advised in dental implant surgery.
Despite the high success rates, failures do happen; they can either be early failures that happen before prosthetic repair or late failures that happen after prosthesis implantation [4]. One potential cause of infection and early implant failure is bacterial contamination at the time of implant implantation [5]. The pathophysiology of peri-implantitis is affected by pathogenic bacteria. It has been discovered that the bacterial flora linked to peri- implantitis, and periodontitis is similar [6]. Consequently, the proposed treatment approach should reduce the microbial burden, clean the implant’s surface, and get rid of any mucosal inflammation around the implant [7]. This strategy would be essential for maintaining the bone support for the implant and starting the bone-regeneration process [7]. In addition to mechanical debridement and oral hygiene instructions, numerous studies have used antiseptics, systemic, or local antimicrobial therapy, and regenerative therapies, as some studies showed that in non-surgical treatment of peri-implantitis, mechanical debridement alone may not be sufficient [8, 9]. As a result, non-surgical methods should only be used to treat peri-implant mucositis because, in cases of bone loss associated with peri-implantitis, the therapy is unable to treat lesions brought on by the inflammatory process [7].
Thus, to effectively remove the granulation tissue and clean the implant surface, surgical treatment is indicated [9]. The goal of using antibiotics in implant dentistry is to employ a bactericidal drug that delays the beginning of infection in the surgical site by increasing the blood concentration of the drug, which will stop bacterial growth and spread. The requirement for antibiotic treatment during implant therapy is debatable because the rate of postimplant placement infection is so low, to begin with (1%–4.4%) [10].
4.1. Antibiotics and Dental Implants Success Rates
Antibiotic administration during dental implant surgery can aid in infection prevention and good wound healing, thereby raising the success rate of dental implants. It is crucial to remember that using antibiotics by itself cannot ensure that dental implants will function properly [11]. The patient’s overall health, oral cleanliness, bone density, and the expertise and experience of the dentist or oral surgeon doing the treatment are all important aspects that affect the effectiveness of dental implants. Patients must carefully follow all post-operative care guidelines, which include maintaining good dental hygiene and abstaining from smoking and other behaviors that could impede healing.
In a systematic literature review conducted in 2012, evaluating 11,406 implants, when no antibiotics were used the success rate was 92% [12], when antibiotic prophylaxis was used the success rate was 96% [12], and when both prophylactic and postsurgical antibiotic coverage was used the success rate was 96% [12]. Another multicenter clinical trial focused on 2,641 dental implants that had been inserted up until the stage II uncovering phase. These were implanted in patients in 1,448 cases who had received prophylactic preoperative antibiotics and in 1,193 cases who had not. Preoperative antibiotic administration resulted in a decrease in failure rates (1.5% vs. 4.0%) [13]. This conclusion is supported by additional studies, which also show that a single loading dose administered before implant surgery had the same benefits as sustained post-implant coverage [14].
4.2. Antibiotics Prophylaxis for Dental Implants
Early dental implant failure, which affects 2% of cases overall, is defined by the implant's failure to osseointegrate [15]. It has been linked to both intraoperative (lack of dental implant stability, implant infection, or trauma during surgery) and postoperative (micro movements exceeding 150 m) causes.
The most frequent bacteria involved are the anaerobic gram-positive cocci, streptococci, and anaerobic gram-negative rods. In particular, strains of Prevotella intermedia, Prevotella nigrescens, Porphyromonas gingivalis, and Actinobacillus actinomycetemcomitans were all identified from infected dental implants and were identical to those seen with chronic periodontal disease [16].
Local infections around dental implants and the likelihood of early implant failure have both been reduced by systemic antibiotic prophylaxis [12, 17]. Yet, there is currently discussion surrounding the use of preventative antibiotics during an implant placement surgical procedure.
Current research has found that in healthy individuals undergoing uncomplicated implant surgeries (defined as procedures without large flaps or concurrent bone regeneration), systemic antibiotic treatment does not significantly minimize early implant failure [18-20]. Moreover, the prescription of systemic antibiotics for local infections after dental implant implantation is ineffective [21].
Yet, several systematic reviews and meta-analyses [5, 20, 22] recommended the administration of a single preoperative antibiotic dose of amoxicillin 1 hour before dental implant surgery. With this approach, the early dental implant failure rate could be lowered to 2% [19]. When compared to preoperative single-dose prophylaxis, perioperative and postoperative antibiotic prophylaxis have shown results that are equal in terms of preventing early dental implant failure [5, 20, 22].
In the case of patients who are at risk of infectious endocarditis, and have a weakened host response, or patients who are having major surgeries that take a long time, or who are having large foreign objects inserted should only be given antibiotic prophylaxis [23]. The most recent recommendations advised taking short-term antibiotic prophylaxis to prevent the beginning of infection at the site of implant placement to raise the antibiotic concentration in the blood and lower the likelihood of bacterial proliferation and dispersion.
Antibiotic prophylaxis has not been proven to improve the results of endosseous implants or prevent postoperative infections, according to studies [24]. If no antibiotics were administered, the risk of post-periodontal surgery infections varies from 1% to 4.4% for periodontal surgery and 4.5% when endosseous implants are placed [10].
The Canadian Dental Association states that antibiotic prophylaxis is typically required for any dental surgical procedures if there is a large amount of bleeding and/or exposure to potentially infected tissue. To avoid bacterial endocarditis, the AHA suggested a program of antibiotics to be given before surgery in 1997. The AHA advises amoxicillin and penicillin as the initial course of treatment due to their better absorption and sustained serum levels. In the event of penicillin allergies, clindamycin is used as an alternative. The classification suggested by Resnick and Misch [25] is useful since it grounds the need for antibiotic coverage on the patient’s medical history, the type of operation, the degree of surgical complication, and the length of the surgery [25] (Table 1).
4.3. Antibiotics and Immediate Implants
Immediate implants placed in sites with apical pathology fail up to three times more frequently than those placed in sites without pathology because of the potential for implant contamination during the early healing period due to the presence of pathogenic bacteria [26]. The authors contend that despite this, urgent implants should always be handled as though the tooth that needs to be taken as a chronic infection because these infections might occasionally be asymptomatic without evident clinical signs of infection, which can lead to implant loss [27-29]. Therefore, it is possible to say that giving prophylactic antibiotics in these circumstances reduces the likelihood of early implant failure [30]. Recommended doses used while placing immediate implants, whether or not the tooth to be extracted has a chronic infection, are shown in Table 2.
4.4. Antibiotics and Peri-implantitis Treatment
Peri-implantitis (PI) is a condition characterized by inflammation and loss of bone and soft tissue around the implant, which can lead to implant failure if left untreated. It is caused by the accumulation of bacteria and microbial biofilm on the implant surface, which triggers an inflammatory response in the surrounding tissues. Other factors that can contribute to the development of peri-implantitis include poor oral hygiene, smoking, diabetes, and certain medications. Symptoms of peri-implantitis may include redness, swelling, bleeding, and discharge from the gums surrounding the implant, as well as loosening or movement of the implant. Prevention of peri-implantitis involves maintaining good oral hygiene, quitting smoking, controlling diabetes, and regular dental check-ups to detect and treat any early signs of the condition. The majority of research shows that the main objective of peri-implantitis therapy is the mechanical removal of bacterial biofilm from the implant surface. Nonetheless, controversial outcomes in the management of peri-implantitis have prompted the consideration of new therapies, including nonsurgical methods such as mechanical instrumentation, antibiotics, antiseptics, chemical or laser decontamination of the dental implant surface, and surgical methods such as air powder abrasion, respective, or regenerative procedures. These methods aim to reduce the microbial load, decontaminate the dental implant surface, and eliminate peri-implant mucosal inflammation to preserve the peri-implant bone [31-36].
| - | Category 1 | Category 2 | Category 3 | Category 4 | Category 4 |
|---|---|---|---|---|---|
| Infection Risk | Low | Moderate | Moderate to High | High | Very High |
| Type of surgery | Simple implant placement (flap or no flap), nongrafted extractions, and second-stage surgery in healthy individuals | Traumatic extractions, socket preservation procedures, and immediate implant placements | Multiple implants with extensive soft-tissue reflection or immediate implant placements along with bone grafting and membrane | Implant surgeries that manipulate or enter the maxillary sinus, autogenous block bone grafts, and the same procedures as in categories 2 and 3 except for medically immune-compromised patients | All lateral wall sinus augmentation procedures. |
| Antibiotics | Not needed | Presurgical antibiotic loading followed by a single dose of antibiotic postsurgical is recommended | Preoperative loading dose of antibiotics followed by three postsurgical doses for a day to be continued for three days | Preoperative loading dose of antibiotics followed by three postsurgical doses for a day to be continued for five days | A loading dose of antibiotics one day before the surgery (ensuring adequate levels in sinus tissues before surgery)and a beta-lactamase antibiotic, penicillin (Augmentin is the best choice), and cephalosporin for five days due to the high incidence of beta-lactamase pathogens in maxillary sinus infections |
| Rinse | 0.12% chlorhexidine (CHX) rinse is recommended pre- and postoperatively | rinse twice a day until sutures are removed | rinse twice a day until sutures are removed | rinse twice a day until sutures are removed | rinse twice a day until sutures are removed and tissue closure |
| - | Antibiotic | Preoperative (I h before) | Postoperative (5-7 days) |
|---|---|---|---|
| Amoxicillin | 2-3 g | 500 mg/8 h | |
| In case of Penicillin allergy | Clindamycin | 600 mg | 300 mg /6 h |
| Metronidazole | 1 g | 500 mg/6 h | |
| Azithromycin | 500 mg | 250 mg/24 h | |
| Clarithromycin | 500 mg | 250 mg/12 h |
4.4.1. Locally Delivered Antibiotics and Peri-implantitis Treatment
Locally delivered antibiotics are an approach that can be used to prevent or treat bacterial infections in dental implants. This involves placing antibiotics directly at the implant site to control the growth of bacteria. This can be done by using antibiotic gels, films, or powders, which are applied during the implant placement or after the implant has been placed. Using locally delivered antibiotics has effectively reduced the risk of infection in dental implants. Clinical studies found that the use of antibiotics delivered locally was associated with a reduced risk of implant failure and improved implant survival rates compared to implants that did not receive antibiotic treatment. To treat peri-implantitis, locally delivered antibiotics were used alone or in conjunction with systemic antibiotics, nonsurgical, and surgical therapies. The most frequently prescribed antibiotics were tetracyclines [7, 37], particularly doxycycline, and minocycline, which are also the most frequently used to treat periodontal infections [38]. Next came metronidazole, which had an advantage over photodynamic therapy [7, 37] when used alone or when combined with amoxicillin, scaling, and root planing [39].
Several studies examined the effectiveness of locally delivered antibiotics alone on periodontal parameters particularly periodontal pocket depth (PPD) [37], bleeding on probing (BoP) [37, 40], and plaque index (PI), the results were inconsistent, probably as a result of the various interventions and control groups used in each study. PPD and BoP were shown to be decreased by local minocycline (“Arestin” in microspheres, “Periocycline” in the ointment), doxycycline gel (“Atridox,” “Ligosan”), lincomycin gel, erythromycin gel, retrocyclin fibers (“Actisite,” and metronidazole gel (“Elyzol”) [37]. Localized minocycline microspheres [7] and 25% metronidazole gel, however, had no beneficial effects on implant failure [41], BoP, PPD, or PI, respectively [7]. Just one research reveals a difference between the treatment group utilizing local antibiotics and the control group in terms of PPD and BoP.
Toledano et al. showed that the local administration of antibiotics favorably impacted the PPD and BoP decrease in peri-implantitis patients. In particular, the probability of bleeding on probing was cut in half when antibiotics were applied topically. Moreover, there was an average 0.30 mm decrease in PPD when antibiotics were treated topically. However, there was no discernible difference between peri-implant measures in minocycline-treated subjects and chlorhexidine-treated subjects [37]. In the trials by Esposito et al., metronidazole was also said to be linked to both improvements in peri-implant conditions and implant failure [41]. The effectiveness of local antibiotics in conjunction with nonsurgical and surgical therapy of peri-implantitis was examined in four investigations.
The various combinations of tetracyclines (doxycycline or minocycline) with nonsurgical treatments (such as scaling and root planing and photodynamic therapy) or surgical flap incision always led to improvement of peri-implant BoP and PPD [7], even at a follow-up of 12 months [40], except in one study in which implant failure was noted [7]. In some instances, gingival index, plaque index [40], and clinical attachment loss [39] are all improved. Similarly, Passarelli et al. discovered that when local antibiotics were added to nonsurgical treatment in comparison to nonsurgical treatment alone, PPD and BoP improved [7].
4.4.2. Systemic Antibiotics and Peri-implantitis Treatment
Systemically given antibiotics have always been assessed in combination with other therapies. However, the use of systemically administered antibiotics in conjunction with non-surgical and surgical treatments for peri-implantitis is still debatable at this time. Nonetheless, there is some evidence to support their usage in peri-implantitis instances that are recurrent and resistant to treatment [42] when different combinations of amoxicillin and metronidazole were used for varying lengths of time and doses along with nonsurgical (such as scaling and root planing, nonsurgical debridement) and surgical therapies with implant debridement only; two trials demonstrated improvements in PPD and BoP [39, 40]. Conversely, Toledano-Osorio et al. found improvements in CAL, suppuration, recession, and total bacterial count in patients with peri-implantitis when using systemically administered antibiotics, but no improvement was seen in BoP and PPD [37].
Similarly, Oen et al. discovered no improvement in PPD when systemic antibiotics and surgical treatments were combined [43]. On the other hand, local in addition to systemic antibiotics have not been shown to have lasting efficacy [44].
CONCLUSION
The widespread use of implants enhances the possibility of peri-implant illnesses in the future. Sadly, there is not much research out there that discusses the therapy of etiological periimplantitis separately. However, enough recent research has shown that in the treatment of peri-implantitis, the removal of the bacterial biofilm from the implant surface, whether through surgical or non-surgical access, should be accompanied by chemical adjunct therapeutic techniques. Unfortunately, there are currently few studies that examine the effectiveness of various chemical therapies used in conjunction with peri-implantitis care. As a result, it is impossible to make a piece of solid advice regarding the chemical adjunct that ought to be employed to treat peri-implantitis.
It is important to note that antibiotics should be used with caution, it should be guided by the patient’s medical history, the severity of the condition, and the type of bacteria causing the infection. The overuse or misuse of antibiotics can contribute to the development of antibiotic-resistant bacteria.
LIST OF ABBREVIATIONS
| CHX | = Chlorhexidine |
| PPD | = Periodontal pocket depth |
| BoP | = Bleeding on probing |
| PI | = Plaque index |
| CTs | = Clinical trials (CTs) |
| SR | = Systematic reviews |
STANDARD OF REPORTING
PRISMA Guideline and methodology were followed.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available in MEDLINE/PubMed, and Cochrane Library databases up to March 2022.


