All published articles of this journal are available on ScienceDirect.
Effect of ER: YAG Laser Treatment on the Bond Strength of Self-adhesive Resin Cements on Contaminated and Non-contaminated Dentin Surface
Abstract
Background:
Nowadays, lasers are used to modify the surface of dentin and enamel and improve the surface bond with resin cement while contamination weakens this bond.
Objective:
This study aimed to evaluate the effect of different contamination types on the microshear bond strength (μSBS) of two self-adhesive resin cements to dentin with/without laser treatment.
Methods:
One-hundred and twenty molar teeth were prepared and randomly divided into three groups: blood, saliva, and control. In the blood group, blood was applied to the samples for 10 seconds; in the saliva group, saliva was applied to the samples for 10 seconds; and in the control group, distilled water was applied to the samples. Each group was further divided into two subgroups; in one, the Er: YAG laser was applied to samples for 5 seconds, and in the other, no laser treatment was performed. Next, TheraCem and Embrace WetBond cements were placed on each sample. μSBS was measured using a universal testing machine.
Results:
In Embrace WetBond, μSBS was not affected by contamination type regardless of laser treatment. In TheraCem, μSBS was not affected by contamination type when the laser was not used. In contrast, with laser treatment, blood contamination decreased μSBS compared to control and saliva. After laser treatment in the presence of blood, μSBS of Embrace WetBond was significantly higher than that of TheraCem, while without laser treatment, no significant difference was observed between the two cements.
Conclusion:
Dentin conditioning with an erbium laser can increase the cement bond strength to dentin if proper isolation is achievable. If proper isolation cannot be obtained, dentin conditioning with erbium laser followed by application of Embrace WetBond cement can increase the microshear bond strength of cement to dentin.
1. INTRODUCTION
In recent decades, the use of indirect ceramic restorations has increased significantly among practitioners due to increased demands for more esthetic restorations [1]. The function of these restorations is most importantly attributed to the luting agent and its bonding ability. Thus, several types of resin cement have been introduced to improve the function of indirect ceramic restorations [2].
An improved bond between the dentin and the restoration decreases future possible problems, including recurrent caries, tooth sensitivity, and marginal discoloration [3]. However, many clinical procedures, including indirect restorations, are not always performed in ideal situations, and thus, contamination with oral fluids should be anticipated, especially in cases in which restoration margins are subgingival and deep or adequate isolation is not achievable [4].
Contamination with blood is considered the most important clinical challenge during restorative treatments [5]. Blood contains 6-7% organic compounds, such as fibrinogen and platelet, which can accumulate on the tooth surface and form a semi-rigid viscous layer interfering with the bonding procedure; this layer might affect the penetration and polymerization of resin cements negatively [4], resulting in decreased bond strength between the tooth and restoration [6].
In addition to blood, contamination with saliva is also common during dental treatments, which results in over-wetting of dentin and contributes to decreasing the bond strength since saliva is composed of 99.5% water and 0.5% organic and inorganic compounds [7]. Moreover, large molecules and glycoproteins in saliva might accumulate on the tooth surface and prevent the adequate bond between the cement and tooth structures [8]. According to the literature, even a short exposure to saliva can result in the formation of a tenacious pellicle on the tooth, which cannot be removed by water rinsing [9]. Additionally, these macromolecules may compete with hydrophilic monomers to penetrate into the dentin, limiting the quality of the bond [10]. It has also been reported that enzymes presented in human saliva are capable of degradation of Bis-GMA monomer presented in many resin-based dental materials, consequently resulting in the breakdown of the resin-tooth interface [11, 12].
Self-adhesive resin cements containing moisture-resistant monomers have recently been introduced to decrease the effect of moisture contamination on the bond strength of resin cements. Self-adhesive resin cements do not require prior preparation of tooth surfaces and have various advantages. Their application is less time-consuming and technique-sensitive, they improve overall aesthetics and have improved mechanical properties and dimensional stability, and they also bond to tooth structures by chemical and micromechanical mechanisms [3, 12]. Little data are presented regarding the effect of dentin contamination on the bond strength of self-adhesive resin cements to dentin. In fact, previous studies are mostly focused on evaluating the bond strength of self-adhesive resin cements to contaminated zirconia ceramic restorations and have concluded that saliva contamination significantly decreased the bond strength of zirconia restorations to dentin cemented with self-adhesive resin cements [13-15]. Moreover, one previous study reported that dentin contamination with saliva could negatively affect the bond strength to resin-luting agents [12]. We could not find any study in which the bond strength of contaminated dentin to self-adhesive resin cements was evaluated.
Embrace WetBond is the first self-adhesive resin cement that contains non-glass ionomer hydrophilic monomers and shows moisture-friendly qualities in the oral environment. This non-irritating and fluoride-releasing cement lacks hydrophobic Bis-GMA monomers, which improves cement hydrophilicity. Moreover, according to the manufacturer, Embrace WetBond does not contain bisphenol A, which might have carcinogenic effects on human cells [16].
TheraCem is a silicate-based cement and bonds to dentin by interactions between mineral ions and resin monomers, forming a micromechanical bond. Its good bond strength and durability have been attributed to its functional MDP-based monomers, which form a durable chemical bond with hydroxyapatite. As a result, an intermediate layer containing two rows of MDP molecules is formed, in which methacrylate molecules are fixed together, and the phosphate groups are placed away from each other. Calcium salts are deposited between the phosphate layers and increase the cement bond strength [17, 18].
Recently, erbium-doped yttrium aluminium garnet laser (Er: YAG) has been used for tooth ablation. In fact, the laser beam is absorbed by the water and OH- groups in hydroxyapatite presented in tooth structure and causes mircoexplosion in water and water-containing organic materials [19]. As a result, this laser might be helpful in removing moisture (saliva and blood) from tooth surfaces and might increase the bond strength of resin cement to the tooth structure. However, results regarding the effectiveness of Er: YAG laser for bond strength improvement in the presence of contamination is controversial. Lepri et al. [20] evaluated the effect of yttrium-aluminum-garnet laser pretreatment on bond strength between contaminated enamel and sealant. They reported that laser application was more effective in improving the bond strength compared to phosphoric acid etching. On the other hand, Moslemi et al. reported no significant improvement in the bond strength of a sealant to contaminated enamel following the laser application [21].
The present study aimed to evaluate the effect of Er: YAG laser application on the bond strength of different moisture-resistance resin cements to dentin in the presence of saliva and blood contamination. The null hypothesis was that micro-shear bond strength values of tested cements, with or without laser treatment and in the presence of different types of contamination, are not significantly different from each other.
2. MATERIALS AND METHODS
In this study, all the methods were carried out in accordance with relevant guidelines and regulations of the Declaration of Helsinki, and the study protocol was registered and approved by the Institutional Research Ethics Committee at the School of Dentistry, Tehran University of Medical Sciences (ethic code: IR.TUMS.DENTISTRY.REC.1398.150). Informed consent was obtained from all the subjects to use their extracted teeth as specimens for this study.
2.1. Tooth Preparation
This in-vitro study used 120 human-extracted third-molar teeth. The teeth were examined using a stereomicroscope (Nikon, SMZ10; Tokyo, Japan) under ×10 magnification to exclude the teeth with caries, hypoplasia, previous restoration, and cracks. The soft tissue residues were removed using a scaler, and the teeth were rinsed with water. The teeth were then disinfected by immersion in chloramine T neutral (0.5%) for 1 week, followed by storage in normal saline at 4°C until use. Afterward, the teeth were vertically mounted in self-cure acrylic resin (Tempron; GC Corp., Tokyo, Japan) using cylindrical molds (3 mm height) so that only the clinical crowns were out of the acrylic resin. To standardize dentinal depth and expose fresh dentinal tubules, the occlusal surfaces of the teeth were ground to a depth of 1 mm within the DEJ using a saw (Isomet; Buehler Ltd., IL, USA) under water irrigation. The samples were then examined using a stereomicroscope under ×20 magnification to ensure the absence of enamel or pulp exposure. Next, the samples were polished manually using 400-600 silicon carbide paper under water irrigation to simulate smear layer formation. Finally, the samples were randomly divided into 3 groups as follows: contamination with saliva, contamination with blood, and dry (control) group.
2.2. Contamination Procedure
Initially, saliva and blood were collected from a single donor in a sterile beaker. For obtaining saliva, the donor was asked to brush and not to eat or drink one hour before saliva collection.
In the first group, saliva was applied to the samples for 10 s using a microbrush and then dried with gentle air flow for 10 s. In the second group, freshly-obtained venous blood was applied to the samples for 10 s using a microbrush and dried with gentle air flow for 10 s. In the third group, the samples were rinsed with distilled water and dried with gentle air flow for 10 seconds. At the end, each group was further subdivided into 2 subgroups, namely A and B, depending on the laser application.
2.3. Laser Application
In subgroup A, the samples’ surfaces were treated using Er: YAG laser without cooling water spray. In the absence of cooling water spray, the laser uses blood or saliva as its mediator. The laser device was used with a power of 1.0 W, frequency of 10 Hz, energy of 100 mJ, pulse intensity of 5897/62 W/cm2, and power density of 353/85 W/cm2. The device tip was placed in a perpendicular direction to the dentin surface at a distance of 1 mm. Each sample received laser treatment for 5 seconds. In subgroup B, the samples did not receive laser treatment.
Next, each subgroup was divided into 2 further subgroups based on the cement type (TheraCem or Embrace WetBond). Table 1 shows the composition of the cements used in the present study.
2.4. Evaluation of Microshear Bond Strength
Cylindrical transparent molds (1 mm in diameter and 3 mm in height) were made from a Tygon tube (MaimeLakes, FL, USA). The molds were placed on the surface of each sample so that each mold was at a 1 mm distance within axial DEJ. Molds were filled with Embrace WetBond and TheraCem cements and were light-cured using a light cure device (Woodpecker, Guilin, China) with 1000 mW/cm2 of power intensity for 40 s. After light-curing, the molds around the cements were cut and removed using a #12 surgical blade. In the end, the diameters of cement blocks were measured using a digital caliper (Mitutoyo Absolute, Kanagawa, Japan) to ensure that all blocks had a diameter of 1 mm. The samples were incubated for 24 h at 37 °C in an incubator (Gallenkamp, Munich, Germany). After the incubation period, the microshear bond strength test was performed using a Universal Testing Machine (Zwick/Roell ZO50, Ulm, Germany) at a cross-head speed of 0.5 mm/min. The following formula was used to calculate the shear bond strength of the samples:
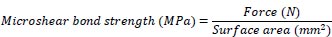 |
2.5. Statistical Analysis
Data were analyzed using SPSS 25, and the significance level was considered 0.05. Due to the normal distribution of data, Three-Way ANOVA was used to examine the effects of cement type, contamination type, and laser treatment on the micro-shear bond strength values and Post Hoc Tukey was performed for pairwise comparison between the groups.
3. RESULTS
The results showed that the cement type and contamination type (blood/saliva) did not significantly affect the microshear bond strength of the cements to dentin (P-values= 0.329, 0.109, respectively). However, laser treatment had a significant effect on the bond strength (P-value=0.009). In addition, there was a significant interaction between the cement, laser, and contamination type (P-value = 0.03), while no significant interaction was observed between laser application and cement type (P-value = 0.06), and also between laser treatment and contamination type (P-value = 0.35).
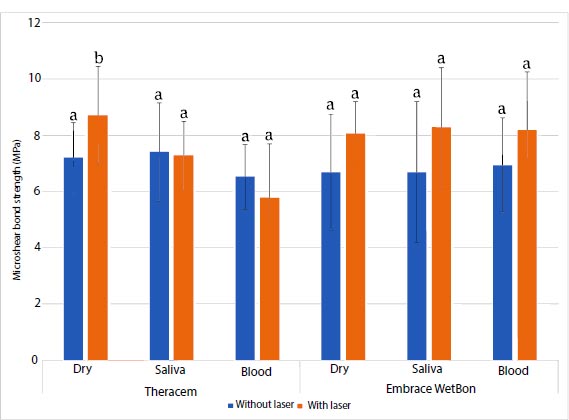
Considering the significant effect of laser treatment on the microshear bond strength values, a T-test analysis was performed. Concerning the cement type, laser treatment increased the microshear bond strength of TheraCem cement regardless of the type of contamination (blood/ saliva/ control); however, the increase was significant only in the control group (P value= 0.038) (Fig. 1). On the other hand, laser treatment decreased the microshear bond strength of Embrace WetBond in all three groups, but the decrease was not statistically significant.
| Material | Manufacturer | Composition | Batch Number |
|---|---|---|---|
| Embrace WetBond | PulpDent USA | Matrix: UEDMA, BMEP, HEMA, TMPTMA, H2O, catalysts Fillers: Barium, glass, ytterbium trifluoride, inert minerals. | Code:228050 |
| TheraCem | Bisco Canada | Base: calcium base filler, glass filler, dimethacrylates, ytterbium fluoride, initiator, amorphous silica Catalyst: glass filler, Methacryloyloxydecyl Dihydrogen Phosphate (MDP), amorphous silica. | Code:43275 |
| - | TheraCem | Embrace WetBond | ||
|---|---|---|---|---|
| Without Laser | With Laser | Without Laser | With Laser | |
| Dry | 7.20 ±1.25aA | 8.72± 1.73aB | 6.69± 2.05aA | 8.06± 1.13aA |
| Saliva | 7.40± 1.74aA | 7.28± 1.23aA | 6.69± 2.51aA | 8.29± 2.12aA |
| Blood | 6.52±1.15aA | 5.79± 0.91bA | 6.49± 1.68aA | 8.18± 2.08aA |
To compare the microshear bond strength of TheraCem and Embrace WetBond, a T-test analysis was performed. After laser treatment in the presence of blood, the microshear bond strength of Embrace WetBond was significantly higher than that of TheraCem (P Value=0.004). However, without laser treatment, no significant difference was observed between the two cements. In saliva and control groups, microshear bond strength values of both types of cement were not significantly different from each other, regardless of laser treatment.
Regarding the effect of contamination type on the microshear bond strength values, in Embrace WetBond, the microshear bond strength was not affected by contamination type, whether or not laser treatment was performed. In the TheraCem group, the microshear bond strength values of the samples without laser treatment were not affected by contamination type. In contrast, in the samples which received laser treatment, the microshear bond strength values were affected by contamination type (P-value=0.0001); blood contamination significantly decreased microshear bond strength compared to control and saliva contamination. No significant difference was observed between saliva contamination and control in this regard.
Table 2 summarizes the effect of laser treatment on the microshear bond strength of dentin to Wetbond and Embrace cements in the presence of blood and saliva, with no contamination.
In each column, the same lowercase letters indicate the lack of significant difference. In each type of cement, in each row, the same lowercase letters indicate the lack of significant difference.
4. DISCUSSION
The present study aimed to evaluate the microshear bond strength of TheraCem and Embrace WetBond cements in the presence of blood/ saliva contamination and laser application. Data showed that without laser treatment, the microshear bond strength of both types of cement was not significantly affected by the contamination type.
Embrace WetBond has an acidic pH, which increases after cement setting, and the set cement has neutral PH. Moreover, the lack of hydrophobic monomers (bisphenol A and its derivatives and Bis-GMA) improved the cement hydrophilicity [22]. In the study conducted by Panigrahi [23], Embrace WetBond sealant exhibited acceptable bond strength in the presence of saliva contamination if it was completely air-dried. This observation might be explained due to the hydrophilic nature of this cement, which allows it to function in the presence of saliva contamination; in fact, cement might be displaced or diffused through moisture and then infiltrate and polymerize.
TheraCem has an acidic pH of about 4 at the beginning of the setting reaction; however, its final pH is 9 after 24 hours. It seems that the microshear bond strength of this cement has not been affected in the presence of saliva or blood contamination due to its acidic monomers. These monomers might hydrolyze and remove contaminations and provide an acceptable bond to dentin. It should be noted that activation of acidic monomers in self-adhesive resin cements requires moisture, and thus, small amounts of moisture are tolerable for this cement [24].
Many studies have used ER: YAG laser to increase the bond strength of composite to dentin [19, 25], while the present study was the first to use erbium laser to remove blood and saliva contaminations from dentin. It is believed that erbium laser needs water molecules to affect dentin and shows a great tendency toward tooth calcified structures, such as hydroxyapatite. Thus, this laser might improve the bond strength of cement to dentin by removing water from the contaminated surface.
There is not sufficient information regarding proper laser parameters for optimized resin–dentin bonding strength during dentin conditioning. One study reported that an erbium laser with an energy of 100 J and frequency of 10 Hz could significantly condition the dentin surface [26]. In both cements, laser treatment increased the bond strength in the absence of contamination; however, this difference was significant only in the TheraCem cement group.
The wavelength of the erbium laser is 2.94 µm, which is close to the absorption peak of water and hydroxyapatite [19]. The water in tooth structures acts as an excellent target for Erbium laser; laser beams are highly absorbed by water molecules in intratubular fluid and collagen fibers, resulting in sudden heating and evaporation of water. The high-stream water pressure causes several micro-explosions in dentin, with the ejection of particles producing a crater-like appearance on the dentin surface [27]. The SEM evaluation of the prepared dentin surface by ER: YAG laser reveals a rough surface free of smear layer open dentinal tubules [28]. Self-adhesive resin cements bond to dentin by chemical adhesion. Laser treatment increases the surface roughness of dentin and forms irregular and microretentive dentinal surfaces. As a result, an irregular dentin surface improves the adaption of self-adhesive resin and, subsequently, increases bond strength. Furthermore, it is believed that roughness increases the available surface for MPD molecules up to 10 times which explains the increased microshear bond strength of cements after laser treatment [28].
Moreover, opened dentinal tubules might affect bond strength. Resin components in cements can easily penetrate opened dentinal tubules. In line with the results of the current study, Munitic et al. [29] and Ulgey et al. [30] concluded that the bond strength of self-adhesive resin cements increased after the application of erbium laser in root canals. According to another study [31], the effect of laser application on the bond strength of cement is attributed to cement composition, which is confirmed by the results of our study. Laser treatment increased the bond strength of Embrace WetBond to dentin in the presence of contamination. However, this increase was not statistically significant. On the other hand, laser treatment decreased the bond strength of TheraCem cement in the presence of blood. It seems that erbium laser coagulated and stabilized blood proteins on the dentin surface, which interfered with the TheraCem functional bonding to dentin.
In contrast, in the presence of saliva, the bond strength was not affected since organic compounds presented only in small amounts in saliva and could not interfere with bonding. The manufacturer has not provided adequate information about monomers in Embrace WetBond cement. Embrace wet bond contains Bis 2-(methacryloyloxy) ethyl phosphate (BMEP) monomer with a phosphate group and 2 polymerizable methacrylate groups. It has a high degree of conversion in the presence of Hydroxyapatite (HA) [32]. BMEP is hydrophile and contains short carbon chains that enhance its wetting and lower the viscosity of the cement so that the cement can easily flow into laser-produced micromechanical spaces in dentin, which explains its increased bond strength to dentin even in the presence of contamination [33, 34]. This result has been confirmed by a previous study [34], which stated that due to the lack of Bis GMA in the Embrace WetBond formula and its lower viscosity, it shows superior marginal adaptation and excellent penetration into dentin. Adhesives containing BMEP are more acidic and hydrophile, and also better wet the surface compared to MDP-containing adhesives and have a higher chemical cure and degree of conversion in the presence of HA [33]. In other words, Embrace WetBond formed a sufficient bond to dentin even in the presence of contamination. In fact, it appears that this cement works better in the presence of moisture, and it can hydrolyze the surface contaminants.
CONCLUSION
Within the limitation of the present study, it can be concluded that dentin conditioning with an erbium laser might increase the cement bond strength to dentin if proper isolation is achievable. If proper isolation cannot be obtained, dentin conditioning with erbium laser followed by application of Embrace WetBond cement might increase the microshear bond strength of cement to dentin.
ABBREVIATION
| μSBS | = Microshear Bond Strength |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study protocol was registered and approved by the Institutional Research Ethics Committee at the School of Dentistry, Tehran University of Medical Sciences (ethic code: IR.TUMS.DENTISTRY.REC.1398.150).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
Informed consent was obtained from all the subjects to use their extracted teeth as specimens for this study.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the corresponding author [S.V] upon reasonable request.
FUNDING
This study was supported by a grant from the Tehran University of Medical Sciences, School of Dentistry, Tehran, Iran (Grant no. 99-1-133-46347).
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest regarding the publication of this article.
ACKNOWLEDGEMENTS
Declared none.


