All published articles of this journal are available on ScienceDirect.
Antibacterial Behavior of Silver Diamine Fluoride, Sodium Hypochlorite and Ozone Gel on Enterococcus faecalis in Root Canal of Deciduous Teeth
Abstract
Introduction:
Enterococcus faecalis (E. faecalis) has been reported as one of the most important causes of failed endodontic treatments. Various antibacterial agents have been suggested to remove E. faecalis from the root canal. We aimed to investigate and compare the antibacterial efficacy of silver diamine fluoride (SDF), Sodium hypochlorite (NaOCl) and ozone gel on E. faecalis in the root canal.
Materials and Methods:
This study involved 60 extracted roots of molar teeth with a single root canal to generate a 6-week-old biofilm for evaluating antibacterial effects. After teeth decorating and removing the smear layer using sodium hypochlorite (2.5%) and EDTA (17%), roots were sterilized by autoclave. Under sterile conditions, 1 ml of the suspension containing E. faecalis bacteria was transferred to each of the microtubes containing teeth. The samples were divided into four groups: exposed to NaOCl (2.5%) as a positive control, exposed to liquid ozone (25 ppm), exposed to SDF (3.8%), and exposed to normal saline as a negative control group. Then, the colony forming unit (CFU) was counted in the studied groups. Kruskal-Wallis test and Dunn-Bonferroni post-hoc analysis were carried out for comparison of CFU in the studied groups.
Results:
The mean of CFU were 17846, 920, 234, and 336 for saline, ozone, NaOCl, and SDF, respectively. There were significant differences in CFU in the comparison of (NaOCl-Ozone), (NaOCl-saline), (SDF-saline), and (ozone-saline) (P< 0.05). However, there was no significant difference for CFU in the comparison of NaOCl-SDF (P= 0.570).
Conclusion:
The study findings showed that NaOCl (2.5%) and SDF (3.8%) were more effective against the 6-week-old E. faecalis biofilms than ozone and saline.
1. INTRODUCTION
Appropriate root treatment maintains the function of deciduous teeth with severe decay [1]. The achievement of a good endodontic treatment in primary teeth is mainly governed by the diminution or removal of bacteria from root canals by antibacterial agents [2]. Although many materials have been suggested for washing and disinfecting the root canal of permanent teeth, the growing, anatomical and physiological variances between deciduous and permanent teeth cause differences in the criteria of the materials used in the stages of root canal pulpectomy [3].
Studies on teeth with failed endodontic treatment have often reported the prevalence of Enterococcus faecalis (E. faecalis), a facultative gram-positive bacterium, in persistent or secondary infections [4]. The invasion of dentin tubules, adaptation to the unfavorable conditions created inside the root canal, and resistance to irrigations and drugs inside the canal are among the reasons for the resistance of this bacteria. Biofilm maturation occurs over time through the mineralization of its structure, and E. faecalis biofilm maturation occurs within six weeks. Then, six weeks is considered as the time index of mature E. faecalis biofilm [5].
The use of sodium hypochlorite (NaOCL), chlorhexidine (in gel or liquid forms), silver diamine fluoride (SDF), and ozone are among the most effective ways to remove E. faecalis from the root canal [6]. NaOCL is the most common agent used for root canal disinfection in concentrations of 0.5 to 5.25% [7]. In most studies, there is no significant difference in the antimicrobial effect of 5% and 2.5% NaOCl [7, 8]. Sodium hypochlorite has disadvantages such as an unpleasant taste, high toxicity, burning surrounding tissues, and inability to remove the smear layer. Another disadvantage of using NaOCl is its unpleasant smell [6, 9].
Due to the disadvantages of NaOCl, the use of other antibacterial materials such as chlorhexidine, ozone, and SDF was also suggested in the previous studies [10-12]. Ozone, as a natural gas, is a strong selective oxidizer. The main foundation of ozone therapy is the rapid decomposition of ozone in water and releases a form of oxygen that may oxidize cells, thus, it has the potential to have an antimicrobial effect without causing drug resistance [13, 14]. Currently, ozone gas is used for adult root canal disinfection and has been demonstrated to be less cytotoxic than 2.5% NaOCl [2].
SDF, as a strong anti-cariogenic material, shows high fluoride release and the ability to remineralize the tooth surface and strengthens its resistency [15]. Although the silver in SDF has an antimicrobial role, its antibacterial effect against root pathogens is poorly understood [16]. Unlike chlorhexidine, SDF has not been shown to be cytotoxic, so it is considered a potential root irrigation [11]. There is limited evidence for the assessment of the antimicrobial action of NaOCl, SDF, and ozone against root pathogens. Therefore, the current study aimed to investigate the antibacterial efficacy of SDF, NaOCl and ozone gel on E. faecalis in the root canal of deciduous teeth.
2. MATERIALS AND METHODS
2.1. Tooth Samples
Sixty-three extracted mesial roots of mandibular molar teeth with a single root canal were used to generate a 6-week-old biofilm model for evaluating the antibacterial efficacy of silver diamine fluoride (SDF), sodium hypochlorite (NaOCl) and ozone gel against E. faecalis in comparison with normal saline. The sample size was determined based on the earlier study [17] by considering the mean±SD (1.29 * 10 ± 0.36) of colony numbers, α= 0.05, and power= 0.80.
2.2. Eligibility Criteria
2.3. Preparation of Dentin Specimens
2.3.1. Preparation of Roots
After debridement of teeth, the samples were sterilized in an autoclave at 121 ºC for 20 min. The roots were sectioned off, applying a diamond disc (D&Z, Diamant, Germany). Then, to split the roots using a low-speed saw (IsoMet 2000 Precision Saw; IsoMet, Buehler, IL) rotating at 1,000 rpm in a buccolingual direction under water cooling was done. Then, mesial roots were selected. Roots with a length of 7 mm were prepared.
2.3.2. Preparation of Canals
The pulp tissue of the canals was removed using a barbed broach (Dentsply, Maillefer, Ballaigues, Switzerland) and the instrumentation of the canals was done by the step-back method.
To remove the smear layer, one ml of 2.5% sodium hypochlorite (Hypo-Endox, Morvabon, Iran) for 1 minute and then one ml of 17% EDTA (Pulpdent Corp, MA, US) for 1 minute were used. Lastly the washing step of roots was done for 1minute with sterile saline The samples were then sterilized by an autoclave at a temperature of 121 ºC for 15 minutes with a pressure of 15 pounds per square inch. Lastly the washing step of roots was done for 1minute with sterile saline [18].
2.3.3. Bacterial Inoculation
The standard strain of Enterococcus faecalis (ATCC 29212) was cultured under sterile conditions on Brain Heart Infusion Agar (QUELAB, Canada) and incubated for 24 hours at 37 ºC. After the incubation period, the half McFarland (1.5 * 10 8 CFU/ML) suspension of the strain was prepared in Brain heart infusion Broth (BHI Broth) (QUELAB, Canada). Under sterile conditions, one milliliter of the prepared concentration was transferred to each of the microtubes containing teeth and incubated for 24 hours at 37 °C. In order to form a mature biofilm for 6 weeks, the suspension inside the micro-tubes was replaced with a new microbial suspension every other day for the purpose of simulating the real conditions of the teeth in the mouth. After 6 weeks, 3 samples were randomly examined to confirm the formation of biofilm under an electronic microscope (LEO s-440i, UK).
2.3.4. The Study Groups
A total of 60 samples were randomly assigned into 4 groups (A, B, C, and D), including 15 samples in each group. The samples were exposed to 0.5 ml of 2.5% sodium hypochlorite as a positive control group (group A), liquid ozone with a concentration of 25 ppm (group B), silver diamine fluoride (SDF) 3.8% (group C), and normal saline as a negative control group (group D), for 1 min. Finally, the canals of all samples in all 4 groups were washed with 10 ml of sterile serum and dried using an aseptic paper cone (DATA, China).
2.3.5. The Number of Colonies: the Colony Forming Unit (CFU)
Sampling was done by Gates Glidden number 4. Then, each of the dental debris was dissolved in 500 microliters (µl) of sterile normal saline and the dilution method was used to count the colony forming unit (CFU) on the same day. The microtube contained 5 µl dissolved debris and 45 µl of normal saline with three concentrations of 10-1, 10-2, and 10-3 were prepared. Then, 10 µl of each prepared concentration was transferred separately to solid agar culture media and spread on the surface of the plate.
After counting the CFU, the obtained number was multiplied by the reciprocal of the selected concentration. Considering that the volume removed for cultivation on the surface of the plate to count the CFU was 10 µL, the obtained number was again multiplied by 100 to obtain the number of CFU in one milliliter (1000 µL).
2.4. Statistical Analysis
For data analysis, we used SPSS software (version 19.0, Chicago, IL, USA). The Kolmogorov-Smirnov test was passed out for testing the normality of data. Descriptive statistics, including mean, Standard Deviation (SD), median, and percentile, were used to present the number of colonies in the study groups. Kruskal-Wallis test was used to compare the between-groups comparison of colonies number since the number of colonies had non-parametric distribution. Dunn-Bonferroni was carried out for post-hoc analysis. The significant level was in P-value <0.05.
3. RESULTS
Overall, 60 extracted mesial roots of mandibular molar teeth were collected; then samples were irrigated with 2.5% sodium hypochlorite (group A), liquid ozone 25 ppm (group B), 3.8% SDF (group C), and normal saline (group D); The step followed by culturing the bacteria that survived after using irrigations., the antimicrobial properties were determined by counting the number of colonies formed and then calculating the number of colonies in one milliliter.
Table 2 shows the mean, median, and descriptive statistics of colonies in the study groups. The mean of CFU in the saline group had a maximum amount, while it had a minimum amount in the NaOCl group. The mean CFU were 17,846, 920, 234, and 336 in salin, ozone, NaOCl, and SDF, respectively. However, the median CFU was zero for NaOCl, and SDF. The mean and SD, and percentiles of the number of colonies are presented in Table 1.
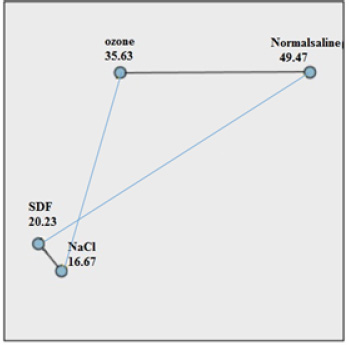
Table 2 and Fig. (1) indicate the comparison of the number of colonies in the studied groups. There was a significant difference in the number of colonies between groups (P= 0.001).
Table 3 shows Dunn-Bonferroni post-hoc analysis of the CFU (pairwise comparison of substances with control groups (NaOCl and saline)). There was a significant difference in the number of colonies (CFU) in two-by-two comparison of groups NaOCl-ozone, NaOCl-saline, SDF-saline, and ozone-Normalsalin (all P< 0.05). However, there were no significant differences for the number colonies in comparison to NaOCl-SDF groups (P= 0.570).
4. DISCUSSION
In the current in-vitro study, the antimicrobial effect of 3.8% SDF, 25 ppm ozone gel, 2.5% sodium hypochlorite and normal saline was investigated on the six-week biofilm of E. faecalis bacteria. The study findings demonstrated that 2.5% NaOCl and 3.8% SDF for 1 minute were the most effective disinfectant protocols against the six-week biofilm of E. faecalis bacteria. Our analysis found that there is no significant difference regarding disinfectant characteristics 2.5% NaOCl in comparison with 3.8% SDF. Furthermore, in several samples, they were able to completely remove 100% of the bacterial biofilm. On the other hand, although the antibacterial effect of 25 ppm ozone was more effective than normal saline, however, normal saline and ozone showed the least effect on the six-week biofilm of E. faecalis bacteria.
E. faecalis is one of the most common microorganisms in infected root canals of deciduous teeth, and is associated with pulp necrosis [19]. Since the bacteria grown in the biofilm are more resistant to antimicrobial treatment than the planktonic bacteria, and the full maturation of the E. faecalis biofilm by its mineralization occurs within six weeks [20, 21]. It is difficult to remove the adult mineralized biofilm of E. faecalis by conventional methods and ultimately leads to resistant and persistent root canal infections [22]. Therefore, the antibacterial protocols were evaluated in this study on the six-week biofilm of E. faecalis. In order to form a mature biofilm for 6 weeks, we replaced the suspension inside the microtubes with a new microbial suspension every other day.
Overall, root canal treatment is considered the most effective way to eliminate inflammation and infection in the root canal, and sometimes, the presence of chronic infection in the root canal may lead to treatment failure [23]. It is almost impossible to remove bacteria using physical methods due to the complications and limitations of the root canal system. The minimum goal of root canal treatment is to reduce the number of bacteria to a level lower than the necessary value for the reproduction of the disease [24]. Irrigations have a major role in pediatric endodontics due to their complex structure and features, such as internal connections and horizontal anastomoses seen in primary teeth [25].
In group A, the samples were exposed to 0.5 ml of 2.5% sodium hypochlorite for 1 minute. Currently, sodium hypochlorite is the most common substance that is used to disinfect the root canal, and it is used in recommended concentrations of 0.5 to 5.25% [7]. There is no agreement regarding the ideal concentration required with sodium hypochlorite to remove bacteria from the root canal. Likewise, the study of Reyhani et al. showed that 2.5% and 5% sodium hypochlorite completely remove the six-week-old biofilm of E. faecalis bacteria. However, as the age of the biofilm increases, its resistance increases compared to 1% sodium hypochlorite [8].
In group B, the samples were exposed to 0.5 ml of liquid ozone with a concentration of 25 ppm for 1 minute. Ozone gel is in oil form in the refrigerator and liquid form at room temperature. Ozonized olive oil gel was chosen for the study because, unlike ozone gas, it does not require expensive devices and the concentration of molecules of ozone is higher and the stability of its composition is also longer [26]. Ozone rapidly breaks down in the water and releases a form of oxygen that may oxidize cells, thus potentially having an antimicrobial effect without causing drug resistance [27]. Regarding the antibacterial effect of ozone, it is able to dissolve debris inside the canal, disinfect parts of the root canal that are not accessible by mechanical tools, be effective against root canal pathogenic microorganisms such as E. faecalis, Candida albicans, Peptostreptococcus, and penetrate through the apical foramen to bone, repair, reconstruction, and wound healing and surrounding bone [15, 28].
In group C, the samples were exposed to 0.5 ml of 3.8% silver diamine fluoride for 1 minute. The SDF was used as an anti-caries agent (38% SDF), which is especially effective in children's dentistry. The SDF is efficient and safe, and it is biocompatible, especially in lower concentrations [29-31]. 3.8% silver diamine fluoride solution was developed for use as an irrigation solution in root canal treatment [32].
One of the mechanisms of the anti-caries function may be caused by the products produced from the reaction between SDF and mineral parts of the tooth. The basic chemical reaction is shown below:
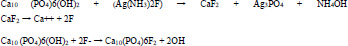 |
The existing fluoride strengthens the resistance of the dentin against the acid, which leads to a decrease in the penetration of the acid into the inner dentin [33]. Also SDF may lead to the hardening of the affected dentin by reacting with dental hydroxyapatite (HA) and releasing calcium fluoride and silver phosphate [34].
The silver of SDF has an antimicrobial role. The antimicrobial role of silver diamine fluoride is related to the formation of organometallic complexes inside bacteria cells. These substances cause the deactivation of enzymes by blocking the electron transport system in bacteria. Also, they cause mutation and death of these cells by interfering with the DNA of bacteria [16].
However, in group D, we used normal saline as a negative control group for comparison of the antibacterial effect in the studied groups.
Generally, the results of our study regarding the antibacterial effect of 3.8% SDF and 2.5% sodium hypochlorite are in line with the results of previous studies.
Al-Madi et al. reported that NaOCl indicated the highest percentage of dead cells, with 62.26%. The SDF group demonstrated a significantly higher percentage of dead cells (57.39%) than the 2% CHX and normal saline groups. They found that SDF had a higher antimicrobial effect than 2% CHX against E. faecalis biofilms [35]. Likewise, Hiraish et al. found that both NaOCl and SDF were effective against E. faecalis biofilms, with no significant difference in reduction of microorganisms for the same exposure time [32]. According to Chaudhari et al. NaOCL had a high effect as a root canal irrigant, followed by SDF and bioactive glass nanoparticle. In that study, the chitosan nanoparticles showed the least antimicrobial effect [36].
Some previous studies evaluated the antibacterial effect of ozone more than what was shown in the current study [13, 37].
In a study conducted by Boch et al. the highest bacterial reduction was found in NaOCl group (99.98%), while EDTA decreased bacteria by 80.64%. Combination of NaOCl and ozone eliminated 99.95% of the bacteria. Combination of EDTA and ozone eradicated E. faecalis up to 91.33% [13]. As a result, ozone gas can dramatically reduce the biofilm of E. faecalis bacteria. In our study, liquid ozone did not have a successful effect in reducing E. faecalis. Possible reasons include the following: 1- Gaseous ozone was used in the above study, while liquid ozone was used in our study. 2- The higher concentration of ozone gas used in Boch et al. study compared to the current study 3- Younger biofilm of E. faecalis bacteria used in their studies compared to the current study.
The findings of the current study are in line with a systematic review by Saliva et al. demonstrated that ozone therapy has meaningfully less microbial effect than NaOCl. They showed that ozone is ineffective in increasing the antimicrobial effect of NaOCl. This finding was powerfully related to the protocol used: the dose, the time and the bacterial strain [38]. However, according to the previous findings, normal saline had the least antimicrobial effect in the present study [39, 40].
5. LIMITATIONS OF THE STUDY
The current study was an in vitro study and its results need to be approved by in vivo or clinical studies. Some issues in sampling could have affected the outcomes. The pure E. faecalis suspension was used in this study, but studies should
| Variables | Study Groups (n=60) | ||||
|---|---|---|---|---|---|
| Normal Saline (n= 15) |
Ozone (n= 15) |
Hypochlorite Sodium (NaCl) (n= 15) |
SDF* (n= 15) |
||
| Mean | 17,846 | 920 | 234 | 336 | |
| Standard Deviation (SD) | 34004 | 837 | 576 | 637 | |
| Median | 3400 | 600 | 0.0 | 0.0 | |
| Percentiles | %25 | 1200 | 0.0 | 0.0 | 0.0 |
| %50 | 3400 | 600 | 0.0 | 0.0 | |
| %75 | 20,000 | 1,400 | 120 | 230 | |
| Groups | Mean Rank | P- value* |
|---|---|---|
| Normal saline | 49.47 | 0.001 |
| Ozone | 35.63 | |
| Hypochloritesodium | 16.67 | |
| SDF | 20.23 |
| Pairwise Comparison of the Groups | Test Statistics | P-value* |
|---|---|---|
| NaCl-SDF | -3.56 | 0.570 |
| NaCl-Ozone | 18.96 | 0.03 |
| NaCl-Normalsalin | 32.80 | 0.001 |
| SDF-Normalsalin | 29.23 | 0.001 |
| Ozone-Normalsalin | 13.83 | 0.028 |
be done concentrating on polymicrobial biofilms rather than individual microorganisms. Some studies reported that SDF may show staining issues after the use of 38% SDF. The concentration of SDF which is used in this study as a root canal irrigator (3.8%), represents a 1:10 dilution of the concentration used for arresting caries (38%). While this subject is not estimated to advance esthetic worries in the case of using this material in molar teeth, this issue should be evaluated in future studies.
CONCLUSION
The study findings showed that 2.5% NaOCl and 3.8% SDF for 1 minute were more effective against the six-week biofilm E. faecalis biofilms than ozone and saline. We found no significant difference regarding disinfectant characteristics of NaOCl 2.5% in comparison with SDF 3.8% in this study.
CLINICAL SIGNIFICANCE
Due to some disadvantages of NaOCL such as burning surrounding tissues and an unpleasant smell, the usage of SDF can be a better clinical option in root canal of deciduous teeth.
LIST OF ABBREVIATIONS
| SDF | = Silver Diamine Fluoride |
| NaOCl | = Sodium Hypochlorite |
| CFU | = Colony Forming Unit |
| SD | = Standard Deviation |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by ethics committee of Tabriz University of Medical Sciences to number: IR.TBZMED.REC.1401.419
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
FUNDING
This study has been funded by the Vice-Chancellor for Research (VCR) of Tabriz University of Medical Sciences (69561).
ACKNOWLEDGEMENTS
Declared none.


