Effect of Chlorhexidine and Ozone Gel on Microleakage and Shear Bond Strength of Dentin Bonding Agents in Composite Resin Restorations of Primary Teeth
Abstract
Aim:
This study aimed to investigate the effects of two antimicrobial agents on shear bond strength and microleakage of composite resin restorations in primary teeth.
Methods:
Sixty extracted primary molars and canines were assigned to three different groups according to the dentin surface pretreatment (2% chlorhexidine gluconate gel, ozone gel, and control group) and two subgroups (sound and caries-affected dentin) for shear bond strength tests. For the microleakage test process, a class V cavity was prepared on the buccal surface of the teeth and restored with composite resin. Then, all the teeth were cut buccolingually. The depth of stain penetration in each specimen was measured separately according to the scale. The data have been analyzed using SPSS 24 software.
Results:
In both sound and caries-affected dentin, ozone gel exhibited higher shear bond strength than chlorhexidine gel and the control group (P<0.001). Chlorhexidine gel had no significant effect on the shear bond strength of sound dentin (P=0.561), but it reduced it in the caries-affected dentin (P<0.001). With both disinfectants, the shear bond strength of the caries-affected dentin was significantly lower than that of the sound dentin. The application of chlorhexidine gel to caries-affected dentin resulted in more microleakage than sound dentin. In addition, the amount of microleakage in the ozone gel group was similar in both types of dentin.
Conclusion:
In both sound and caries-affected dentin, ozone gel was associated with higher shear bond strength than chlorhexidine gel; furthermore, chlorhexidine increased microleakage and ozone gel decreased it.
1. INTRODUCTION
Conventional techniques for treating dentin caries include completely removing carious tissue to reach a well-mineralized structure. This method can lead to pulp exposure and the need for more complex treatments in unnecessary cases [1].
More conservative techniques are now being developed to preserve pulp vitality and remineralize dentin [2]. Removing caries to reach the affected dentin by preserving the vitality of the pulp reduces the use of local anesthetics and the pain and discomfort of the patient; most importantly, it decreases the treatment time, which is crucial in pediatric dentistry [3]. During cavity preparation for an adhesive composite resin restoration, caries-infected dentin is removed. Large areas of the cavity floor are composed of caries-affected dentin; therefore, in the clinical setting, the bonding substrate is usually caries-affected dentin, not sound dentin, which has many structural differences [4]. Therefore, the cavity walls contaminated by bacteria due to caries are a problem in restorative dentistry [5] because bacteria can remain and multiply in the smear layer or dentinal tubules. Studies have shown that the proliferation of residual bacteria in the smear layer releases bacterial toxins into the pulp, leading to pulp irritation and inflammation [5, 6].
The microorganisms present in the tooth cavity wall are not eliminated by water spray or dentin bonding agents containing disinfectants [7]. Therefore, after preparing the cavity, an antibacterial solution is required to successfully eliminate the cariogenic bacteria and prevent the release of toxins by the bacteria, which leads to postoperative sensitivity following composite resin restorations [8, 9]. Previous studies in this field indicate that ideal dentin disinfectants should be able to eliminate bacteria and not adversely affect the strength and stability of bonding systems [10]. Cavity disinfectants may affect the bond strength of adhesive restorations. The main concern regarding oxidants, such as ozone, while placing adhesive composite resin restorations is adverse effects on adhesion due to the inhibition of polymerization of monomers [11, 12].
A study in Turkey conducted in 2013 [13] on the effect of chlorhexidine and ozone gas on microleakage and tensile bond strength in compomer restorations in normal deciduous teeth showed no significant difference in bond strength between ozone gas and the control group. However, the chlorhexidine group exhibited significantly lower bond strength. Concerning microleakage, there were no significant differences between the three groups.
As reported in the research carried out in Turkey in 2018 [14] on the effect of chlorhexidine and ozone on the shear bond strength of composite resin to dentin in permanent teeth, chlorhexidine did not adversely affect the shear bond strength. Therefore, it was suggested as a disinfectant before restoration. However, ozone reduced the shear bond strength of dentin.
The structure and composition of dentin are different in deciduous and permanent teeth. For example, primary teeth have lower amounts of calcium and phosphate in peritubular and intertubular dentin and fewer dentinal tubules [15]. In addition, primary teeth have a thinner enamel layer than permanent teeth. Therefore, the results of studies on permanent teeth cannot be generalized to deciduous teeth.
Accordingly, and because we did not find any similar study on the effect of chlorhexidine and ozone gel on the shear bond strength and microleakage of composite resin restorations in deciduous teeth, we carried out this in vitro study to evaluate the effect of two common and effective antimicrobial agents on cariogenic bacteria and the bond quality of composite resin restorations.
2. MATERIALS AND METHODS
This in vitro study was performed at the Pediatric Dentistry Department, Faculty of Dentistry, Tabriz University of Medical Sciences, from December 2021 to March 2022, and approved by the Ethics Committee of the Tabriz University of Medical Sciences (reference number: IR.TBZMED.REC.1400. 843). 60 extracted first and second molars and 120 extracted deciduous canines (including sound and decayed) teeth were collected. The samples included 60 teeth in the shear bond strength test group and 120 in the microleakage test group. The teeth were collected near the start of the experimental phase and stored in a sterile physiological saline solution at 4ºC. All the samples were incubated in 0.2% thymol solution for disinfection for 48 hours.
2.1. Shear Bond Strength Test
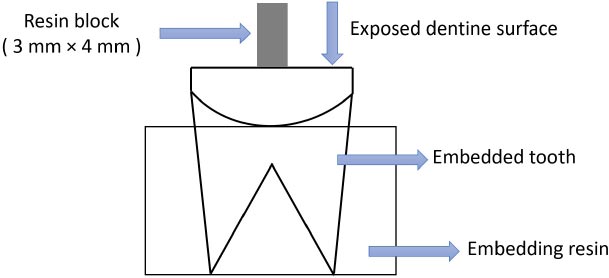
For the shear bond strength test, the samples were cut mesiodistally and perpendicular to the longitudinal axis with a diamond disk and water spray. The exposed dentin surfaces were further flattened using wet 600-grit silicon carbide paper for 60 seconds to standardize the smear layer. The teeth were then rinsed with distilled water to remove debris and randomly divided into three groups and two subgroups (sound and decay dentin) of 10 teeth each. In the case of the preparation of caries-affected dentin specimens, the caries was removed after the procedures described below using a caries detection dye. Then, it was cut in the mesiodistal direction, and the surface was prepared with sandpaper for 30 seconds to achieve a smooth and standardized surface in all the samples. The technique to stain dentin explained by Fusayama was used to distinguish remineralized dentin from demineralized dentin [4]. A caries-detecting dye (Sable Seek Caries Indicate, UltraDent, USA) was used to determine carious dentin. A low-speed handpiece, a round carbide bur (Teeskavan.CI.023), and water spray were used to remove stained dentin. The diagnostic dye was used again and rinsed, and the stained dentin was removed. This continued until the dentin was no longer stained, and finally, the dentin surface was finished using wet 600-grit Si-C papers to create a standard smear layer. The occlusal surfaces of caries-free teeth were also prepared to expose the dentin and were used as sound dentin groups [3]. All the teeth were then placed in 3×2-cm cylindrical plastic molds containing self-curing acrylic resin so that the roots were embedded in acrylic resin. The samples were restored according to the manufacturer’s instructions for each material (Figs. 1, 2).
2.2. Experimental Design
60 teeth were randomly selected, and the samples were divided into three different groups, with 20 teeth in each group. Each group was then subdivided into two subgroups: sound and caries-affected dentin (10 teeth in each subgroup). The samples were prepared for the shear strength test as follows:
1. The teeth were disinfected with ozone gel (Vitomix) for 40 s and then rinsed and dried with air for 10 s.
2. The teeth were disinfected with chlorhexidine digluconate gel (2% chlorhexidine gel, Morvabone, Iran) (Fig. 3) for 30 seconds, rinsed, and dried with air for 10 s.
3. Teeth were treated as control group, in which no disinfectant was applied [13].
2.3. Bonding Procedures
The adhesive system was applied following the manufacturer’s instructions [13]. Thus, phosphoric acid (37% Morva-Etch, Iran) was applied to dentin for a maximum of 15 s, rinsed with water for at least 10s, and blot-dried with a moist cotton pellet [16]. Prime and bond NT (Dentsply Sirona, USA) was applied to sound and caries-affected dentin, which was placed on the surface for 20 seconds, dried for 5 s, and light-cured for 10 s with an LED light source (Guilin Woodpecker, China; 1100 mW/cm2). Opus Bulk Fill (FGM, Brazil) composite resin was applied to the dentin surface and light-cured for 40 s using the same light-curing unit. Transparent gelatin capsules (3 mm in diameter, 4 mm in height) were used to form and hold the restorative resin onto the dentin surface. It was kept in distilled water at 37ºC for 24 h [17]. To simulate the condition of the oral cavity, the thermocycling method was performed using a thermocycling machine, which included 500 cycles at 6/60°C for 30 s each time. The samples were kept in distilled water at room temperature until all samples were prepared for the shear bond strength test [18]. The shear bond strength of the restoration was evaluated by applying a force perpendicular to the longitudinal axis of the tooth using a universal testing machine (Hounsfield H5K-S, UK). The knife section of the device was placed parallel to the tooth material interface. The compressive force was increased at a crosshead speed of 0.05 inches/min and continued to increase until the restoration was displaced. The results were obtained as Newton and transformed to MPa by dividing these by the surface area [19, 20].
2.4. Microleakage Test
On the buccal surface of the teeth, a class V cavity was prepared using a cylindrical diamond bur in a high-speed handpiece under air and water spray [13]. Each cavity was created 1 mm above the cementoenamel junction and was approximately 2 mm wide, 2 mm deep, and 3 mm long. Each preparation was rinsed with distilled water for 20 s and dried with compressed air for 20 s [13].
| Microleakage Scores | |
|---|---|
| 0 | No dye penetration at all |
| 1 | Dye penetration up to half of the cavity wall |
| 2 | Dye penetration to the whole cavity wall |
| 3 | Dye penetration to the cavity walls and floor |
| 4 | Dye penetration partially or totally reaching the pulp |
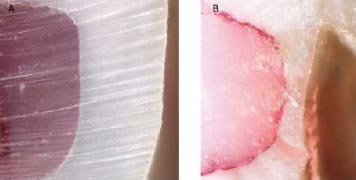
All the samples were selected and prepared, similar to the method described in the shear bond strength test. The cavity margins were finished with a 30-blade composite resin carbide finishing bur (Teeskavan; needle length: 012) and aluminum-coated disks (Sof-Lex, 3M ESPE, St. Paul, MN, USA) 24 h after the completion of the restoration. All the teeth were immersed in distilled water for 24 h at 37°C. Then, the samples were covered with acid-resistant nail polish 1 mm beyond the restoration outlines. The apex of the teeth was sealed by glass-ionomer (Meron; VOCO, Cuxhaven, Germany). The samples were incubated in 0.5% fuchsine solution at 37°C for 24 h. Afterward, the teeth were rinsed to remove the excess dye. Then the teeth were cut buccallingually almost at the center of the restoration using an IsoMet low-speed saw [13].
Two observers separately examined the microleakage at the incisal and gingival margins under a stereomicroscope (Nikon CDS-Japan) at ×40 magnification (Fig. 4). In each sample, the penetration depth of the stain was measured separately, as shown in Table 1 [13].
3. RESULTS
3.1. Determination and Comparison of Shear Bond Strengths of Dentin Bonding Agents in Composite Resin Restorations
Since the shear strength data were distributed normally (Kolmogorov-Smirnov; Z=0.479 and P=0.979), parametric tests were used to evaluate it in three types of dental cavity disinfectants in two types of dentin. Two-way ANOVA showed the effects of dentin and the antiseptic solution on shear band strength to be significant (P<0.001), but the cumulative effect of antiseptic solution*dentin was not significant (P=0.079). Next, we examined each of the main effects (Table 2).
| Source | Type III Sum of Squares | df | Mean Square | F | P-value |
|---|---|---|---|---|---|
| Corrected model | 1863.343 | 5 | 372.669 | 28.689 | <0.001 |
| Intercept | 20262.113 | 1 | 20262.113 | 1.560E3 | <0.001 |
| Dentin | 447.174 | 1 | 447.174 | 34.424 | <0.001 |
| Disinfectant gel | 1346.085 | 2 | 673.043 | 51.812 | <0.001 |
| Disinfectant*dentin | 70.084 | 2 | 35.042 | 2.698 | 0.076 |
| Error | 701.464 | 54 | 12.990 | - | - |
| Total | 22826.920 | 60 | - | - | - |
| Corrected total | 2564.807 | 59 | - | - | - |
| - | Caries-affected Dentin(n=30) | Sound Dentin(n=30) | P-value | ||
| Mean | SD | Mean | SD | ||
| Control | 15.72 | 3.47 | 19.26 | 2.34 | 0.016 |
| Chlorhexidine | 8.83 | 2.23 | 17.31 | 6.15 | 0.001 |
| Ozone | 22.39 | 2.79 | 26.75 | 3.13 | 0.004 |
| P-value* | <0.001 | <0.001 | - | ||
| (I) | (J) | Sound Dentin | Caries-affected Dentin | ||
|---|---|---|---|---|---|
| Difference in Mean(I-J) | P-value | Difference in Mean(I-J) | P-value | ||
| Control | Chlorhexidine | 1.9500 | 0.561 | 6.8900 | <0.001 |
| Control | Ozone | -7.4900 | 0.001 | -6.6700 | <0.001 |
| Chlorhexidine | Ozone | -9.4400 | <0.001 | -13.560* | <0.001 |
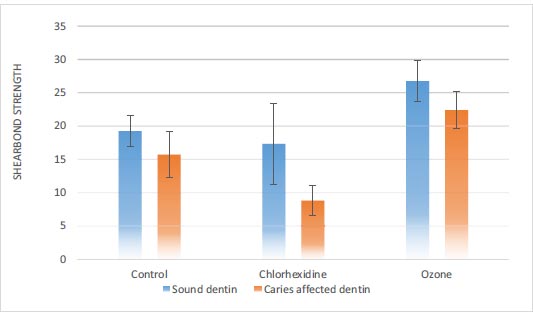
3.1.1. Sound Dentin
The results showed a significant difference in shear bond strength in terms of the type of disinfectant gel in sound dentin (P<0.001) (Table 3). The shear bond strength in the ozone gel group was significantly higher than in the chlorhexidine gel and control groups. However, the shear bond strength in the chlorhexidine gel and the control group was similar (Table 4).
3.1.2. Caries-affected Dentin
In caries-affected dentin, there was a significant difference in shear bond strength in terms of the type of disinfectant gel (P<0.001) (Table 3). The shear bond strength in the ozone gel group was significantly higher than the chlorhexidine gel and control groups. The shear bond strength of chlorhexidine gel was significantly lower than the control group (Table 4).
The results also showed that in all three control, chlorhexidine gel, and ozone gel groups, the shear strength of the caries-affected dentin was significantly lower than that of the sound dentin (P<0.05) (Fig. 5).
The percentages of the fracture modes observed in all tested groups are presented in Table 5. The principle mode of failure of the bond in the sound dentin, control group, and chlorhexidine group was adhesive, and in the ozone group, it was cohesive. The principle mode of failure of the bond in the caries-affected dentin, control group, and chlorhexidine group was adhesive, and in the ozone group, it was cohesive. The mixed type was the least-observed type of failure mode among the groups. The results, thus, showed a significant difference in the frequency of fractures in both types of dentin.
| - | Sound Dentin | - | - | Affected Dentin | - | - |
|---|---|---|---|---|---|---|
| - | Adhesive | Cohesive | Mixed | Adhesive | Cohesive | Mixed |
| Control | 4(40%) | 4(40%) | 2(20%) | 4(40%) | 4(40%) | 2(20%) |
| Chlorhexidine | 4(40%) | 5(50%) | 1(10%) | 5(50%) | 4(40%) | 1(10%) |
| Ozone | 2(20%) | 7(70%) | 1(10%) | 1(10%) | 7(70%) | 2(20%) |
| P-value* | 0.034 | - | - | 0.015 | - | - |
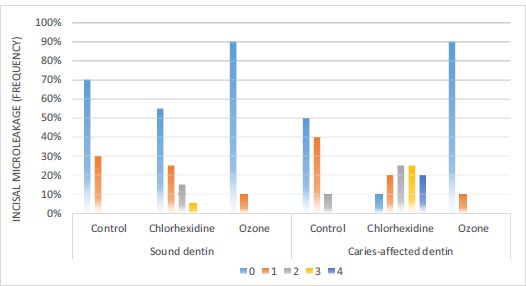
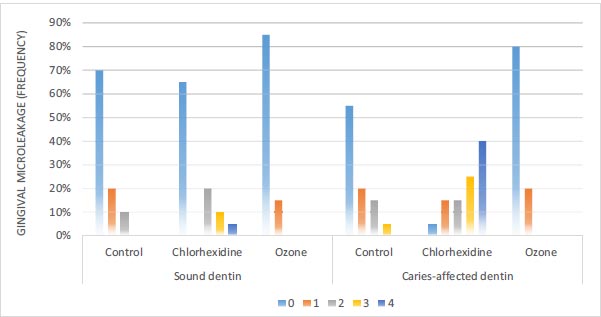
3.2. Determination and Comparison of the Microleakage of Dentin Bonding Agents in Composite Resin Restorations of Deciduous Teeth
3.2.1. Incisal Microleakage
According to Fig. (6), in sound dentin of the ozone disinfectant, chlorhexidine disinfectant, and control groups, 90%, 55%, and 70% of the samples had no incisal leakage, respectively. Based on the results of the Chi-squared test, there was a significant difference in the amount of incisal microleakage between the three groups (P=0.048). In caries-affected dentin of the ozone disinfectant, chlorhexidine disinfectant, and control groups, 90%, 10%, and 50% of the samples had no leakage, respectively. Based on the results of the Chi-squared test, there were significant differences in incisal microleakage between the three groups (P<0.001) (Fig. 2).
| - | - | Control | Chlorhexidine | Ozone | |||
|---|---|---|---|---|---|---|---|
| Incisal | Gingival | Incisal | Gingival | Incisal | Gingival | ||
| Sound Dentin | 0 | 70.00% | 70.00% | 55.00% | 65.00% | 90.00% | 85.00% |
| 1 | 30.00% | 20.00% | 25.00% | - | 10.00% | 15.00% | |
| 2 | - | 10.00% | 15.00% | 20.00% | - | - | |
| 3 | - | - | 5.00% | 10.00% | - | - | |
| 4 | - | - | - | 5.00% | - | - | |
| P-value | 0.301 | 0.156 | 0.500* | ||||
| Caries-affectedDentin | 0 | 50.00% | 55.00% | 10.00% | 5.00% | 90.00% | 80.00% |
| 1 | 40.00% | 20.00% | 20.00% | 15.00% | 10.00% | 20.00% | |
| 2 | 10.00% | 15.00% | 25.00% | 15.00% | - | - | |
| 3 | - | 5.00% | 25.00% | 25.00% | - | - | |
| 4 | - | - | 20.00% | 40.00% | - | - | |
| - | P-value | 0.466 | 0.679 | 0.331* | |||
3.2.2. Gingival Microleakage
According to Fig. (7), in sound dentin of the ozone disinfectant, chlorhexidine disinfectant, and control groups, 85%, 65%, and 70% of the samples had no gingival leakage, respectively. Based on the Chi-squared test, there were no significant differences in gingival microleakage between the three groups (P=0.074). In caries-affected dentin of the ozone disinfectant, chlorhexidine disinfectant, and control groups, 80%, 5%, and 55% of the samples had no gingival leakage, respectively. According to the Chi-squared test, there were significant differences in gingival microleakage between the three groups (P<0.001) (Fig. 3).
Table 6 shows that in both sound and caries-affected dentin of all three control, ozone, and chlorhexidine groups, the amount of incisal and gingival leakage was similar, with no significant difference.
4. DISCUSSION
4.1. Shear Bond Strength
In the present study, there was a significant difference in the shear bond strength in sound dentin based on the type of disinfectant solution (P<0.001). The shear bond strength in the ozone gel group was significantly higher than in the chlorhexidine gel and control groups, with no significant difference between the chlorhexidine gel and control groups. In caries-affected dentin, there was a significant difference in the shear bond strength in terms of the type of disinfectant solution (P<0.001). The shear bond strength in the ozone gel group was significantly higher than in the chlorhexidine gel and control groups; the shear bond strength in the chlorhexidine gel group was significantly lower than in the control group. Also, in all three types of disinfectants, the shear bond strength of caries-affected dentin was significantly lower than that of sound dentin.
Kapdan et al. [13] investigated the effect of using cavity disinfectants consisting of 2% chlorhexidine and gaseous ozone on dentin bond strength in compomer restorations of deciduous teeth. They found that the bond strength of the resin when cleaning the cavity with chlorhexidine was significantly lower than when ozone was used. They reported that the high activity and oxidation potential of ozone might have affected some physical properties of dentin. Therefore, the bond strength values were higher in the ozone group than in the control group. Cangul et al. [20] evaluated ozone as a cavity disinfectant along with three types of adhesive systems and compared it with the control group. They concluded that the ozone applied to the cavity before permanent tooth restoration increased the bond strength of adhesive materials and killed bacteria. Ercan et al. [21] reported ozone as the best cavity disinfectant among three disinfectants (ozone, chlorhexidine, and boric acid) and the use of ozone did not demonstrate an adverse effect on the shear bond strength of the composite resin. In their study, the highest bond strength values were recorded in the control group, and the ozone group did not differ from the control group. However, the groups treated with chlorhexidine and boric acid demonstrated significantly lower values than the control group. The results of the above studies are consistent with the present study, as in the present study, the use of ozone increased the shear bond strength more than chlorhexidine in both sound and caries-affected dentin. However, some studies have shown results different from the present study. In this regard, Yavuz et al. [14] investigated the effects of chlorhexidine and ozone on bond strength in permanent teeth, reporting that chlorhexidine did not affect the shear bond strength of restorative materials. However, bond strength in the ozone group was significantly lower than in controls.
Arslan et al. [22] reported that the use of the tested cavity disinfection agents, chlorhexidine and ozone, did not significantly affect the dentin bond strength of a silorane-based resin composite. Cadenaro et al. [23] evaluated the effects of gaseous ozone application on enamel and dentin bond strength and compared two self-adhesive systems (Clearfil Protect Bond and Xeno III), reporting that the use of ozone gas for cavity disinfection before bonding procedures did not affect the immediate bond strength of enamel and dentin.
Ozone gas is a natural disinfectant that has been widely used due to its strong antibacterial, antiviral, and antifungal effects, and wound-healing properties. Ozone gas has an unstable structure and is rapidly converted to oxygen, and it is the only natural antibacterial agent that leaves no residues after disinfecting the cavity [24]. Polydorou et al. [14] concluded that the application of ozone might be effective in eliminating bacteria remaining under restorations, especially S. mutans and Lactobacillus casei, two essential bacteria responsible for tooth decay. Ozone, a strong disinfectant, can react with all organic material and reduce the wettability of dentin surfaces, preventing plaque formation [20]. The present study showed that in sound dentin, the shear bond strength was similar in the chlorhexidine gel and control groups. However, in caries-affected dentin, the shear bond strength in the chlorhexidine gel group was significantly lower than that in the control group. Chlorhexidine is currently one of the most common cavity disinfectants in clinical practice. Its popularity can be attributed to the effectiveness of its antibacterial effect and its ability to function as a matrix metalloproteinase (MMP) inhibitor [25]. Hassan et al. [9] reported that chlorhexidine-disinfected dentin had higher shear bond strength than the control group in permanent teeth, unlike the present study. Studies by Mobarak et al., de Castro et al., Ricci et al., and Shafiei and Memarpour found that chlorhexidine did not affect the shear bond strength of etch-and-rinse bonding systems [26-29]. Ebrahimi et al. [30] also showed the positive effect of chlorhexidine on increasing the shear bond strength of self-etching systems to dentin in deciduous teeth, with no positive effect in etch-and-rinse systems.
Many studies have concluded that chlorhexidine may act as a rewetting or protective agent, effectively regulating the structural integrity of collagen materials [31]. One study investigated the effect of chlorhexidine on some self-etching systems, suggesting that the substance used may directly affect how chlorhexidine and adhesives interact [29]. In the present study, in caries-affected dentin, shear bond strength in the chlorhexidine gel group was significantly lower than that in the control group, possibly because chlorhexidine can bind to apatite phosphate groups on the smear layer on the dentin surface due to its cationic property and since it has a negative effect on resin infiltration [14]. The difference between the results of previous studies and the present study may be explained by differences in the methods of using disinfectants, for example, applying before the etching procedure or after that, and rinsing or not rinsing the antimicrobial agent. In addition, the form of the material (gel or solution) and the duration of use may affect the results. Another reason is the difference in the type of teeth examined due to the differences in microstructure and mineral composition of permanent and deciduous teeth.
4.2. Microleakage
Microleakage is defined as the clinically undetectable diffusion of bacteria and toxins, oral fluids, molecules, and ions through the gaps between the cavity walls and the restorative material. The most important factor for the long-term clinical success of adhesive restorations is establishing an effective and permanent connection between the restorative material and the tooth surfaces. Due to the shrinkage during the polymerization of restorative materials, micro-cracks are created between the tooth and the restoration. Bacteria, ions, and fluids can easily pass through these gaps, causing secondary decay, pulp inflammation, postoperative sensitivity, and margin discoloration. In the present study, in each of the two types of dentin (sound and caries-affected), the amount of incisal microleakage was significantly different in the three groups, with the least microleakage in the ozone gel group and the highest in the chlorhexidine group. In sound dentin, the amount of gingival microleakage was not significantly different between the three groups. However, in caries-affected dentin, the amount of gingival leakage was significantly different in the three groups. The lowest microleakage was observed in the ozone gel group, and the highest microleakage was observed in the chlorhexidine group. In both dentin types (sound and caries-affected), chlorhexidine gel and ozone gel had a similar effect on incisal and gingival leakage. The results of the present study also showed that the application of chlorhexidine gel to caries-affected dentin caused more leakage than sound dentin. However, with the ozone gel, the amount of leakage was similar in both dentin types.
Consistent with the present study, Güneş et al. [32], in an in vitro study, evaluated the effects of ozone gas, laser, and traditional cavity disinfectants on microleakage and found that the minimum leakage was recorded in the ozone group. In 2019, Bahrololoomi et al. [33] investigated the effect of two disinfectants on the microleakage of resin-based composite restorations in deciduous teeth. Disinfection with chlorhexidine and sodium hypochlorite increased microleakage in composite resin restorations of deciduous teeth. There was also no statistically significant difference in the gingival and incisal surfaces, consistent with the present study. In a study by Kapdan et al. [13], the amount of occlusal and gingival microleakage was similar in the chlorhexidine and gaseous ozone groups in compomer restorations in deciduous teeth. However, Cellik and Bahsi [34] reported different results. In their study, in both ozone and chlorhexidine groups, the amount of microleakage in the gingival margins was significantly higher than in the occlusal margins, and the highest microleakage was seen at the gingival margin. In a study by Salama et al. on resin-based composite restorations in deciduous teeth, there was no significant difference between the microleakage of the three groups of sodium hypochlorite, chlorhexidine, and persica. The cervical margin also showed more microleakage than the incisal edge. The samples used in this study had mild to moderate caries that affected the microleakage score [35].
The differences in the results can be explained by the variety of adhesives, different statistical analyses, types and concentrations of antimicrobial agents, and the operator’s experience. Microleakage studies of restorative adhesives applied to the cervical region have shown that gingival margins have higher microleakage than occlusal margins due to the thinness or, in some cases, the absence of the enamel layer in the cervical region and the positive effect of the thicker enamel layer on the margin microleakage [32]. In addition, since the gingival margins of the cavities are located in the cementum, the cementum-dentin junction has a more permeable structure, compared to enamel, which may lead to more stain penetration in the gingival margin, as shown in many studies [13]. Considering these data, the application of ozone can be an effective alternative to the existing methods of eliminating oral cariogenic bacteria. The study showed that ozone applied to the cavity before restoration increased the bond strength and decreased the microleakage of adhesive agents. However, further in vivo and in vitro studies are necessary to confirm these findings. However, as in other studies, there were some limitations in the present study too, including the lack of aging processes in the samples. It is suggested that further studies be carried out with higher concentrations and more antibacterial agents. For the carious teeth, the inclusion criteria were dentinal caries extended no further than the middle one-third of the dentin thickness, as verified by the radiograph [36]. Then, the teeth underwent a 500-round thermocycling process (30 s each) at 5/55°C [37]. Based on some studies, it has been suggested that oxygen remaining on the dentin surface may be responsible for the reduction in bonding strength of resin materials [38, 39].
CONCLUSION
In sound and caries-affected dentin, ozone gel positively affected the shear bond strength and microleakage score. However, chlorhexidine gel had an adverse effect on shear bond strength and incisal microleakage only in caries-affected dentin.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This in vitro study was performed at the Pediatric Dentistry Department, Faculty of Dentistry, Tabriz University of Medical Sciences, from December 2021 to March 2022, and approved by the Ethics Committee of the Tabriz University of Medical Sciences (reference number: IR.TBZMED.REC.1400. 843).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee, and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
Informed consent was obtained from all participants.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article will b available upon request from the corresponding author [ A. S].
FUNDING
None.


