All published articles of this journal are available on ScienceDirect.
Effect of Glutaraldehyde on Dentin Hypersensitivity after Non-surgical Periodontal Therapy: A Randomized, Triple-blinded Clinical Study
Abstract
Aims and Objectives:
The purpose of this study was to evaluate the effect of 5% glutaraldehyde (5% Gluma), in association with 37% phosphoric acid conditioning, on dentin hypersensitivity (DH) after non-surgical periodontal treatment (NSPT). Additionally, we investigated the impact of these treatments on health-related quality of life (HRQoL).
Methods:
Ninety hypersensitive teeth of subjects were randomized into the following three groups based on treatment (n = 30): GP: placebo gel that simulates 37% phosphoric acid (37% PA) + distilled water, GPG: placebo gel that simulates 37% PA + 5% Gluma, and GAG: 37% PA + 5% Gluma. Two visual analog scales (VASs) were used to measure DH after tactile and evaporative stimulation four times. The HRQoL was recorded using a DH experience questionnaire (DHEQ). Statistical analysis of DH data was performed using Friedman vs. Kruskal-Wallis tests. DHEQ data were analyzed using Wilcoxon vs. Mann–Whitney tests and a simple logistic regression (α= 0.05).
Results:
The GPG and GAG groups showed significantly lower DH than GP (p ≤ 0.05) for tactile and evaporative stimulations. After one month of follow-up, the GPG and GAG groups showed an increase in HRQoL, which was higher than that of the GP group (p ≤ 0.05). Gluma (5%) effectively prevented DH for up to 15 days after NSPT, regardless of previous conditioning with phosphoric acid. In addition, treatment with 5% gluma had a positive impact on HRQoL.
Conclusion:
The treatment with 5% glutaraldehyde was effective in HD and had a positive impact on quality of life.
Clinical Trial Registration Number:
The clinical trial registration number for this study is NCT04207450.
1. INTRODUCTION
Quality of life may be negatively affected by the oral diseases assessed by the health-related quality of life (HRQoL) [1, 2]. Periodontal diseases, characterized by symptoms, such as bleeding and pain, significantly affect the HRQoL of individuals [3]. Previous studies have shown that suboptimal oral health affects self-esteem, quality of life, and general well-being [4, 5]. A systematic review conducted by Botelho et al. showed that non-surgical periodontal treatment (NSPT) improved HRQoL within a short period, and it was stable after 3 months of treatment [6].
Technological advances have facilitated the advent of several protocols for use in NSPT, including the use of ultrasound [7], lasers [8], photodynamic therapy [9], and the administration of antimicrobials [10]. However, they did not show significant benefits compared to the NSPT by hand curettes. Therefore, the debridement protocol depends on the preferences of the clinician and the patient [11]. In dental clinics, the Gracey curettes are the most used for mechanical removal of calculi and bacterial biofilms during NSPT [12].
Clinical studies have shown a high prevalence of dentin hypersensitivity (DH) in patients undergoing periodontal procedures [13-15]. Several theories have been proposed to explain DH [16-18]. However, the hydrodynamic theory proposed by Brännström is the most widely accepted [18]. External stimuli, mostly thermal or mechanical, generate fluid movement in the dentinal tubules, which causes pain due to the stimulation of the A-δ fibers [19]. According to this mechanism, the dentinal tubules must be obliterated so that the surface stimuli do not result in the movement of intra-tubular fluids [20, 21]. Several protocols and materials for preventing DH after NSPT have been tested [20-24], but no gold standard has been established to date. In addition, no recent systematic review is available in the literature. Therefore, more randomized clinical studies with a low risk of bias are required.
A systematic review evaluated the efficacy of desensitizing agents and showed that glutaraldehyde has high efficacy for immediate DH reduction [25]. However, the study assessed DH, which was not related to NSPT. The gluma desensitizer (Heraeus Kulzer GmbH, Wehreim, Germany) is a desensitizing agent consisting of a 5% aqueous solution of glutaraldehyde and 35% 2-hydroxyethyl methacrylate [26, 27]. The application of glutaraldehyde to hypersensitive dentin leads to the coagulation of proteins inside the dentinal tubules; this obliterates the dentinal tubules and reduces DH [28]. Although the manufacturer of the gluma desensitizer indicates that the material should be applied over clean dentin without any type of acid etching, this study hypothesized that the removal of the smear layer might enhance the penetrability of glutaraldehyde. To the best of our knowledge, no previous clinical trial has evaluated the effect of gluma desensitizer in volunteers with DH following NSPT. This lack of research supports the need to conduct this study.
Therefore, the main objective of this randomized, triple-blinded, placebo-controlled clinical study was to evaluate the effect of 5% gluma desensitizer in association with 37% phosphoric acid (37% PA) conditioning on dentinal hypersensitivity after NSPT and its durability after 15 and 30 days of treatment. We also investigated the impact of this treatment on the HRQoL of the volunteers. The null hypotheses tested in the present study were as follows: H01: There will be no statistically significant decrease in DH after non-surgical periodontal treatment with 5% gluma desensitizer with or without 37% PA, and H02: There will be no difference between the improvements in HRQoL of the participants before and after NSPT following treatment with 5% gluma desensitizer with or without 37% PA.
2. MATERIALS AND METHODS
2.1. Trial Design and Study Participants
This was a placebo-controlled, triple-blinded, randomized clinical trial conducted from May, 2021, to December, 2021. Comparisons between three groups were made with two exposed groups to the intervention. Volunteers of both sexes aged between 18 and 50 years who sought dental care at the integrated clinic of the local dental school were included in the study.
2.2. Ethical Aspects
This randomized, parallel, triple-blinded, placebo-controlled, single-center clinical study followed the recommendations of CONSORT (Consolidated Standards of Reporting Trials) [29, 30] and its extension, CONSORT PRO (Patient Reported Outcomes) [31]. This study was approved by the Human Research Ethics Committee of the Institute of Health Sciences of the Federal University of Pará with the CAAE number 01089918.3.0000.0018 and registered on the clinical trial registration website, ClinicalTrials.gov, with the identifier NCT04207450. After adequate education on the risks, methods, and objectives of this study, all research participants signed the free and informed consent form in accordance with the Declaration of Helsinki [32].
2.3. Sample Size
To define the sample size of the study, the BioEstat 5.3® program (Belem, Para, Brazil) was used. The sample calculation was performed through a pilot study involving seven volunteers (30 hypersensitive teeth) under the same conditions and criteria as the present study. The number of teeth was considered the sample unit instead of the number of subjects. A difference of three units between the baseline and the final VAS score was considered clinically relevant. With the data from the pilot study, 80% power was adopted, and 24 teeth were required for each group. Adjusting for a 20% loss, 28 teeth per group were finalized. The total number of teeth used in this study was 90. The limit of significance was set at 5%.
2.4. Inclusion and Exclusion Criteria
All participants included in this study were examined and selected based on the following inclusion criteria: volunteers with at least 2 teeth with gingival recession with or without a periodontal pocket of up to 5 mm of probing depth, which presented with painful sensitivity response to tactile and evaporative stimuli at levels ≥ 4 on the VAS ranging from 0 to 10, where 0 and 10 represented no pain and extreme pain, respectively, and ≥ 2 on the Schiff scale (0 to 3) for the evaporative test. The exclusion criteria were patients with systemic diseases, pulpitis, carious lesions, presence of restorations on selected teeth, cracked enamel, non-carious cervical lesions, use of medication with analgesics and/or anti-inflammatory drugs, pregnant or lactating women, desensitizing treatment, and NSPT received during the three months before the study recruitment.
2.5. Randomization and Allocation
A simple numerical draw was carried out using numbered and coded papers that allowed teeth to allocate themselves to one of the three groups. The code for each group was unknown to the volunteers, surgeon, and evaluator. The allocation secrecy was maintained throughout the sampling process, and only one of the study collaborators (F.A.S) was aware of the treatments.
2.6. Blinding
In this triple-blinded study, all volunteers were unaware of the treatment they received. The 5% aqueous solution of glutaraldehyde was mimicked with distilled water, and 37% PA was mimicked with placebo gel (based on glycerin and natural dye). They were all presented in similar containers, which made differentiating them impossible. The compounds used as placebo showed viscosity and color similar to the experimental compounds.
Like the volunteers, the DH examiner was also unaware of the group of patients, as he did not participate in the process of randomization, allocation, and intervention. The clinical trial had a single surgeon who performed the experimental part and was unaware of the compound used in the treatment. Thus, the study was triple-blinded.
2.7. Non-surgical Periodontal Treatment
The selected volunteers underwent scaling and root planing (SRP) procedures with curettes (Millennium - Golgram, São Caetano do Sul, SP, Brazil) in the affected regions: vestibular, palatal, and interproximal. The scraping was performed by removing the entire mineralized stone structure until a smooth surface was observed with the number 5 explorer probe (Millennium - Golgram, São Caetano do Sul, SP, Brazil).
2.8. Intervention
Desensitizing treatments were performed after the SRP protocol according to the group. Details on the group allocation of patients are shown in Fig. (1).
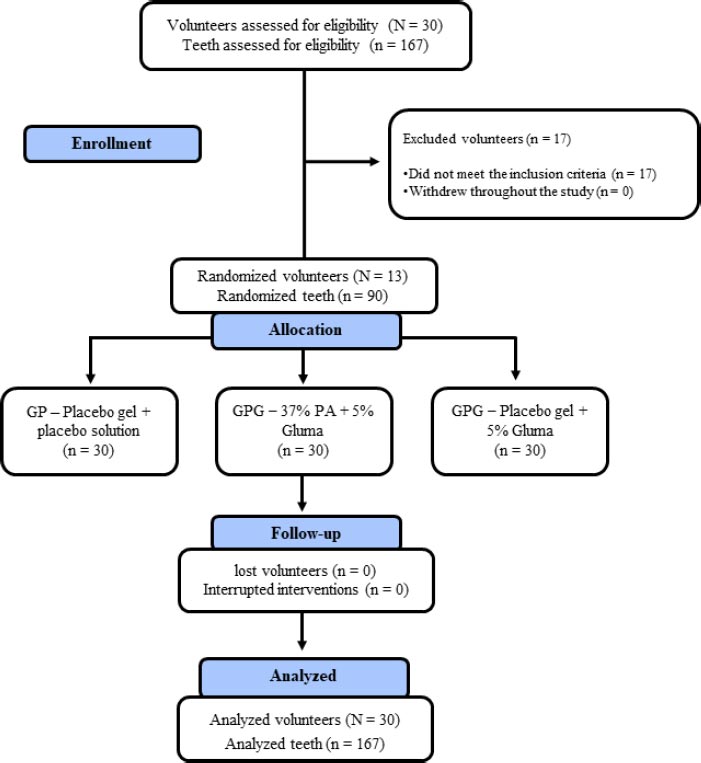
1) GP (Placebo group): The selected teeth were isolated with a Gingival Barrier Top Dam-FGM (FGM, Joinville, SC, Brazil) to prevent contact between the soft tissues and the desensitizing agent. After that, the placebo gel was applied to simulate the application of 37% PA for 15 seconds. The teeth were rinsed thoroughly and dried with sterile absorbent paper. Subsequently, distilled water was applied to simulate the application of 5% gluma. Once again, the teeth were allowed to dry for 30 to 60 seconds, and a light jet of air was applied. Finally, the surfaces were rinsed abundantly, thus, mimicking the application of the gluma desensitizer (Kulzer, São Paulo, SP, Brazil).
2) GPG (placebo gel + 5% Gluma group): As in GP, all selected teeth were isolated with a gingival barrier. After that, the placebo gel was applied as in GP, followed by the application of the 5% gluma solution, i.e., the gluma desensitizer (Kulzer, São Paulo, SP, Brazil) with the aid of a microbrush applicator (FGM, Joinville, SC, Brazil). The desensitizer was left on the surface of the teeth for 30 to 60 seconds, and a light jet of air was applied until the liquid dried and the surface lost its luster. Finally, the surfaces were thoroughly rinsed.
3) GAG (37% PA + 5% Gluma group): The gingival barrier was applied as in GP. After that, conditioning with 37% PA (condac37, FGM, Joinville, SC, Brazil) was performed for 15 seconds. Copious washing was performed for 30 seconds, and the conditioned surfaces were dried with sterile absorbent paper, leaving the dentin moist. Subsequently, the gluma desensitizer was applied in a similar way to the GPG group.
All volunteers received a kit containing a brush with light bristles (Curaprox, São Caetano do Sul, SP, Brazil), dental floss (Oral B, São Bernardo, SP, Brazil), and fluoride-free toothpaste or any desensitizing agent (Malvatrikids Turiaçu, Rio de Janeiro, RJ, Brazil). Oral hygiene instructions were given for standardization to prevent interference with the results.
2.9. Assessment of Dentin Hypersensitivity
DH assessments were performed in four stages: after SRP scraping, after desensitizing treatment, 15 days after SRP, and 30 days after SRP. The sensitivity of dental elements was assessed using tactile and evaporative stimulation and VAS (0 to 10). The VAS values were interpreted as follows: 0 = absent pain, 1 – 3 = mild pain, 4 – 6 = moderate pain, and 7 – 10 = severe pain. First, the tactile stimulus test was performed; the exploratory probe 5 (Quinelato, Rio Claro, SP, Brazil) was placed in contact with the dentin from apical to incisal and mesial to distal after SRP. Subsequently, an evaporative stimulus test was performed using a 40 psi air jet of the triple syringe (Croma T5 Cart, Dabi Atlante, Ribeirão Preto, SP, Brazil) for 3 seconds. Simultaneously, the evaluator quantified DH based on the expression of pain by the patient using the Schiff scale. During all tests, neighboring teeth were protected with cotton rolls. The Schiff scale was proposed by Thomas Schiff in 2009 [33] and was scored as follows: 0- Tooth/patient does not respond to the stimulus; 1- Tooth/patient responds to the stimulus but does not require discontinuation of the stimulation; 2- Tooth/patient responds to the stimulus and requires interruption or moves from the stimulation; 3- Tooth/patient responds to the stimulus, expresses pain through words, and requires discontinuation of the stimulation.
Teeth that presented moderate DH, ≥ 4 on the VAS, and a response ≥ 2 on the Schiff scale were included in the research. Those with pain below 4 on VAS and less than 2 on the Schiff scale were excluded from the study.
2.10. Dentin Hypersensitivity Experience Questionnaire (DHEQ)
A self-reported questionnaire was administered to the volunteers to capture individual experiences 24 hours and 1 month after SRP to assess the effect of desensitizing treatment on HRQoL. The DHEQ questionnaire used was adapted from Douglas-De-Oliveira et al. (2018) [34]. The short version of the instrument consists of 15 questions that assess the impact of DH on HRQoL on five subscales: functional restrictions, adaptation, social impact, and emotional and personal identity.
2.11. Statistical Analysis
The SPSS statistical program (SPSS Statistics 25.0, IBM) was used for statistical analysis. A normality test was performed for the DH data corresponding to the VAS using the Shapiro-Wilk test. Non-parametric tests were used for both VAS and the Schiff scale (Friedman vs. Kruskal-Wallis). The Friedman test was used for intragroup analysis, and the Mann-Whitney test was conducted for the intergroup evaluation of independent samples. Dunn's post-test was used to detect differences. All analyses considered significance levels of 5%. In addition, the tooth and non-individual sample units were considered. In this cluster data, the subject was included as a random effect.
DHEQ data were analyzed using Wilcoxon and Mann–Whitney tests. The effect of DH treatment on HRQoL was assessed using simple logistic regression. The differences between the final DHEQ data and initial DHEQ data were transformed into binary data. Values ≥ 5 were considered as worsening and ≤ 3 as improvement in DHEQ. The final average of VAS with an evaporative stimulus > 4 was considered ineffective and ≤ 3 as effective in the treatment of DH. This analysis was adapted from Ortiz et al. (2019) [35].
3. RESULTS
The demographic characteristics of the 13 participants and 90 teeth (N=13; n=90) included in this research are presented in Table 1.
3.1. Assessment of Dentin Hypersensitivity
The results presented in Table 2 show that the VAS scores for DH were significantly higher immediately after SRP than during evaluation periods (p ≤ 0.05) for the tactile and evaporative stimuli. After treatment, GPG and GAG had significantly milder DH than GP (p ≤ 0.05) for tactile and evaporative stimulation. Fifteen days after SRP, there was no statistical difference between the groups studied for tactile stimulation; however, there was a statistically significant difference between the experimental groups and GP (p ≤ 0.05) for the evaporative stimulation. In addition, there was no statistically significant difference between GPG and GAG during all periods of assessment for both stimuli (p > 0.05).
The results presented in Table 3 show that the Schiff scale scores for DH were significantly higher immediately after SRP than during other evaluation periods (p ≤ 0.05) for the evaporative stimulation. After treatment, the experimental groups showed significantly milder DH than GP (p ≤ 0.05). There was no statistically significant difference between GPG and GAG (p > 0.05).
| Categories | GP (N=4/n=30) | GPG (N=5/n=30) | GAG (N=4/n=30) | P |
|---|---|---|---|---|
| Gender N(%) | ||||
| Female | 2(50) | 4(80) | 3(75) | *0.062 |
| Male | 2(50) | 1(20) | 1(25) | - |
| Age, Years | ||||
| Man Interval |
34,2 29-46 |
30 22-42 |
28 22-33 |
**0.002 |
| Tooth Type, n(%) | ||||
| Incisors | 10(33.33) | 17(56.66) | 15(50.00) | *§0.862 |
| Canines | 4(13.33) | 4(13.33) | 3(10.00) | - |
| Premolars | 11(36.66) | 8(26.66) | 7(23.33) | - |
| Molars | 5(16.66) | 1(3.33) | 5(16.66) | - |
**ANOVA test;
*§ Kruskal-Wallis test.
| GROUPS | EVA Tactile Stimulation - M (±ID) | |||
|---|---|---|---|---|
| - | After SRP | After Treatment | After 15 days | After 30 days |
| GP | 7.2 (± 3.7)Aa | 5.2 (± 2.6)Aa | 2.2 (± 5.0)Bb | 0.5 (± 2.0)Bb |
| GPG | 6.3 (± 2.1)Aa | 0.5 (± 2.3)Bb | 1.5 (± 2.7)Bb | 0.5 (± 3.0)Bb |
| GAG | 7.5 (± 2.0)Aa | 0.0 (± 0.5)Bb | 0.0 (± 2.0)Bb | 0.0 (± 1.0)Bb |
| Evaporative Stimulation EVA - M (±ID) | ||||
| After SRP | After treatment | After 15 days | After 30 days | |
| GP | 8.7 (± 3.4)Aa | 7.1 (± 3.2)Aa | 6.0 (± 3.7)Aa | 1.0 (± 2.7)Bb |
| GPG | 7.5 (± 2.5)Aa | 2.6 (± 2.0)Bb | 0.0 (± 3.5)Bb | 0.5 (± 3.4)Bb |
| GAG | 8.2 (± 3.8)Aa | 0.0 (± 3.5)Bb | 0.0 (± 2.2)Bb | 0.0 (± 1.1)Bb |
* Different lower-case letters represent statistically significant inter-group differences (p≤0.05).
| GROUPS | Evaporative Stimulation Schiff - M (±ID) | |||
|---|---|---|---|---|
| - | After SRP | After Treatment | After 15 Days | After 30 Days |
| GP | 3.2 (± 1.1)Aa | 3.0 (± 1.0)Aa | 2.2 (± 1.7)Aa | 1.0 (± 2.0)Bb |
| GPG | 3.1 (± 1.0)Aa | 0.5 (± 1.0)Bb | 0.5 (± 1.1)Bb | 0.0 (± 1.0)Bb |
| GAG | 2.9 (± 0.5)Aa | 0.0 (± 1.2)Bb | 0.0 (± 1.0)Bb | 0.0 (± 1.0)Bb |
* Different lower-case letters represent statistically significant inter-group differences (p≤0.05).
3.2. Dentin Hypersensitivity Experience Questionnaire (DHEQ)
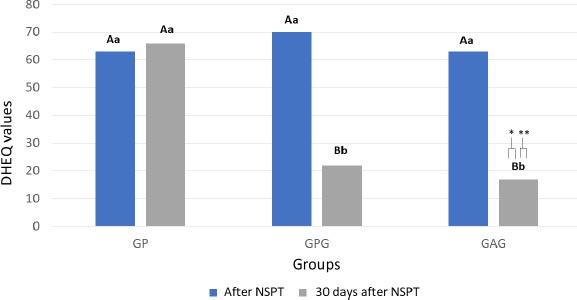
A statistically significant difference was found between DHEQ data before and one month after treatment (p = 0.022). The intragroup analysis showed no difference between the DHEQ values before and 30 days after treatment in GP (p = 0.678). The GPG and GAG groups showed a significant improvement in HRQoL (p = 0.018 and p = 0.001, respectively) after 30 days of treatment. The intergroup analysis showed that the DHEQ medians at baseline were similar for all groups (p = 0.872). After a month of follow-up, the GPG and GAG had lower DHEQ values, which correspond to an improvement in HRQoL, than GP [Z = 2.983 (p = 0.002); Z = 2.982 (p = 0.008), respectively] (Fig. 2).
3.3. Impact of Desensitizing Treatment on HRQoL
Simple logistic regression analysis showed a relationship of dependence between the HRQoL improvement and the desensitizing treatment effectiveness after a one-month follow-up. An effective desensitizing treatment has an 87.36% (p = 0.026) chance of improving the HRQoL.
4. DISCUSSION
There is no desensitizing protocol recommended in the literature to be applied after non-surgical SRP. Commonly, in the dental clinic, no desensitizing treatment is applied to the sensitive dentin before or after SRP. In addition, it is known that the excessive wear of cementum and dentin during SRP can lead to exposure of the dentin tubules, which is the main cause of DH. The mechanical loss of hard tissue is related to several etiological factors, including gingival recession, surgical therapy, scaling, root planing, and in most cases, their combinations [36]. However, the results of the present study demonstrated that the proposed treatment with 5% gluma was effective in DH with a positive impact on patients' quality of life. Therefore, hypotheses H01 and H02 were rejected.
A meta-analysis showed that the prevalence of DH may reach an average of 33.5% in adults [37]. However, a systematic review conducted by Draenert et al. (2013) [36] on DH following SRP concluded that there were no adequate randomized studies in the literature, and it was found that the weakness of all epidemiological studies is the lack of objective measures for measuring pain. Therefore, it is necessary to conduct further research before making specific recommendations for the correlation between periodontal disease and dentin hypersensitivity.
The results of the present clinical study showed that 5% gluma prevented DH after non-surgical SRP, regardless of previous conditioning with 37% PA, for up to 15 days after SRP. However, 30 days after SRP, there was no significant pain sensitivity in any of the groups studied. Previous studies have shown that sensitivity gradually decreased a few days after periodontal treatment, possibly due to a natural mechanism of desensitization [38, 39]. The protein-rich biofilm layer, or salivary film, is formed during the first two hours of salivary exposure and protects against acid challenges and significant resistance to demineralization of dental tissues [40]. The formation of the salivary film may be related to a decrease in DH in the short term after SRP.
Twenty-four hours after SRP, followed by desensitizing treatment, DH significantly reduced in GPG and GAG, regardless of the stimulus and scale used. This is due to the obliterating mechanism of action of the gluma desensitizer. Previous studies have shown that gluma is a potent DH inhibitor, which is capable of obliterating dentinal tubules and penetrating up to 200 µm deep into them [41, 42]. The mechanism of action of this material occurs by the reaction between glutaraldehyde and plasmatic proteins of dentin causing protein precipitation and consequent dentinal obliteration [42]. On the other hand, previous conditioning with 37% PA did not significantly influence the manifestation of DH throughout the study. The mechanism of action of glutaraldehyde may not be influenced by the conditioning of hypersensitive dentin.
The evaporative stimulus was associated with lesser painful sensitivity in the experimental groups than in the placebo group for up to 15 days after SRP. However, the tactile stimulus was associated with milder DH in the experimental groups for only up to 24 hours after SRP. Possibly, the tactile stimulation was less sensitive for the detection of DH than the evaporative. This may be attributed to the difficulty of standardizing the passage of the explorer probe over the hypersensitive dentin; the probe can easily deviate on the curved surface of the cervical third and change the load applied under it. In addition, not all of the exposed dentin surfaces involve areas with DH, and areas with DH may change between evaluations [43, 44].
The air-jet test is considered the most accurate method for assessing DH because it involves a wider area of dentin, in addition to being used more frequently than the tactile or thermal tests in clinical trials [45]. The DH patient is susceptible to all types of painful stimulation daily, which is why it is recommended to perform more than one form of pain assessment [46]. In this clinical study, the Schiff scale used for the evaporative stimulation was used in addition to the VAS. This evaluation was carried out to help the evaluator's assessment of the volunteers' painful manifestation [33].
In addition to measuring DH compared to the proposed treatments, this clinical trial showed that desensitizing treatments had a positive effect on the volunteers' quality of life. The DHEQ has excellent psychometric properties with adequate reliability (general correlation> 0.4; Cronbach's alpha = 0.86) and validity (r = 0.92) to accurately assess functional and personal changes in patients with DH [47]. DH is a condition that can significantly interfere with the daily lives of affected individuals [34]. Therefore, DH prevention strategies must become permanent after periodontal treatments.
Although the baseline characteristics of the participants demonstrated homogeneity of the sample, with regard to the strict eligibility criteria, the variability of the teeth included in the study (incisors, canines, premolars, and molars) may have affected the manifestation of DH, which is a strength of the study. This can be considered a limitation of this study, despite all the standardization measures taken. Although two scales (VAS and Schiff) for quantifying pain have been used, the perception of DH is subjective, as it is subjected to the individual questions of the participants, which is a weakness of the study. It is known that verbal reports are shaped by several psychosocial variables. Pain is not a simple sensory state, but it is influenced by the cultural context, situation, level of attention, and other psychological variables [48].
CONCLUSION
The results of the present clinical study showed that 5% gluma prevented DH after non-surgical SRP, regardless of previous conditioning with 37% PA, for up to 15 days after SRP and had a positive impact on the quality of life of the individuals evaluated.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The research ethics committee of the Institute of Health Sciences of the Federal University of Pará reviewed and approved the trial (Approval no. 01089918.3.0000.0018).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures were followed in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the corresponding author, [C.M.S.], on special request.
FUNDING
This work was supported by the National Council for Scientific and Technological Development and the Federal University of Pará for granting scholarship number (Finance Code 001) for the development of the research.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the National Council for Scientific and Technological Development (CNPq) and the Federal University of Pará for their support in the development of research.


