All published articles of this journal are available on ScienceDirect.
The Cell Biocompatibility of a new Polycaprolactone-based Endodontics Sealer Compared with AH Plus Sealer
Abstract
Background:
One of the important materials needed for root treatment is the sealers. They fill the space between the gutta and the canal walls and create a suitable seal to prevent the colonization of oral microorganisms in the peri-apical tissues and inside the canal space.
Aim:
The aim of this study was to prepare and test the cell biocompatibility of a new polycaprolactone-based endodontics sealer in comparison to AH plus sealer against dental pulp stem cells.
Methods:
MTT assay was used to evaluate the cytotoxicity.
Results:
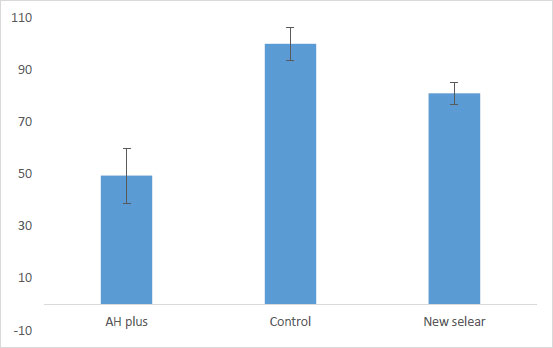
The results showed that AH plus showed moderate cytotoxicity effects against cells (the number of living cells was 49.45 ± 10.56%). However, for the new sealer, the number of living cells was ≥ 80% (81% ± 4.11). Thus, the sample was non-cytotoxic against tested cells.
Conclusion:
More investigations are needed on the new sealer to validate its biocompatibility and usefulness.
1. INTRODUCTION
Effective root canal treatment is mediated by correct diagnosis, effective cleaning, and proper filling. In dentistry, root canal treatment is performed in order to fill the root canal and eliminate complications inside the canal. For a successful treatment, the canal must be completely filled and cleaned of all infectious microorganisms [1, 2]. One of the important materials needed for root treatment is the sealers. Sealers fill the space between the gutta and the canal walls, and create a suitable seal to prevent the colonization of oral microorganisms in the peri-apical tissues and inside the canal space [3]. A good sealer should have characteristics of lubrication, radio-opaqueness, compatibility with oral tissue, antimicrobial property, solubility, working time, toxicity, dimensional stability, and adhesion to the components inside the canal [4, 5].
Bacteria and the substances produced by them are the main etiological factors for diseases inside the canal [6]. For this reason, the main goal of root canal treatments is to remove microorganisms from inside the root canal and prevent secondary infections [7]. Despite the mechanical canal fillings, antibacterial washing, correct filling of the canal, and the use of an antibacterial coating, such as calcium hydroxide, between root canal treatment sessions, an infection-free environment cannot be created [8]. For this reason, ideal root treatment materials should be bacteriostatic and prevent the growth of bacteria [9].
Different types of sealers have different bases, including zinc oxide, eugenol, calcium hydroxide, glass ionomer, epoxy resin, silicone base, and bioceramics. AH+ sealer, which is a modified combination of an epoxy resin sealer called AH26, has better tissue compatibility and involves less color change due to the absence of silver, compared to AH26 [10, 11].
In a recent study, the cytotoxicity of four types of root canal treatment sealers was compared using human gingival fibroblasts. In that study, all sealers exhibited cytotoxicity [12]. Besides, in another study, some commercial sealers showed different degrees of toxicity to the studied cells [13].
Resin-based sealers are the most common and well-known functional sealers in contemporary endodontics, which can be used both laterally and vertically. In the past, eugenol base sealers were more common. Sealers with medicinal base, sealers with calcium hydroxide base, glass ionomer, etc., have been introduced in the past, but they have never been as reliable and popular as resin sealers, both in terms of study and commercial results [14]. Polycaprolactone is a very biocompatible synthetic polymer that is widely used in medical, pharmaceutical, and dental applications. This basic material is considered excellent for the production of new resins in endodontics [14].
Considering the high cost of sealers and the cytotoxicity of commercial sealers, the design of sealers with a lower total cost, which at the same time have favorable physicochemical, physicomechanical, and antimicrobial properties, can be one of the research paths in science. Therefore, the preparation and cytotoxicity of a new polycaprolactone-based resin were studied and compared with commercial AH plus sealer (as one of the updated sealers).
2. METHODS
2.1. Sealer Preparation
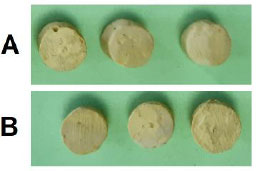
The new sealer proposed in this study was based on polycaprolactone resin sealers. To prepare the sealer, caprolactone methacryloxy ethyl ester (CMEE) powders, bioactive glass, zinc oxide, barium sulfate, calcium hydroxide, calcium phosphate, and zirconium oxide were mixed according to the percentages presented in Table 1 by the spatulation method. Mixing was continued until the powders have been properly mixed. Also, in order to homogenize the mixture, the desired mixture was sonicated for one hour in an ultrasonic bath. Polycaprolactone resin (P767 and P787) was heated to a temperature of 70°C, and the rest of the powders were dissolved in it to be completely combined with polycaprolactone in order to form a homogeneous paste. Then, the disks (6 mm in diameter) were prepared from the sealer samples. The disks for AH plus sealer (as one of the updated sealers) were also prepared as a control group, as shown in Fig. (1).
| Material Name | Percentage |
|---|---|
| P767 | 30 |
| P787 | 5 |
| Caprolactone methacryloxy ethyl ester (CMEE) | 10 |
| Bioactive glass | 10 |
| Zinc oxide | 10 |
| Barium sulfate | 15 |
| Calcium hydroxide | 2.5 |
| Calcium phosphate | 15 |
| Zirconium oxide | 2.5 |
2.2. Assessment of the Cytotoxicity
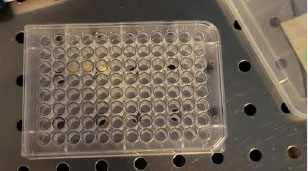
In this research, dental pulp stem cells were used for cell culture to investigate the toxic effect of sealer. In order to perform these experiments, the cells were purchased from Shahid Beheshti University (Tehran, Iran). The prepared sealer was placed in the bottom of the wells, and then the cells were cultured in a single layer in DMEM containing serum and antibiotics. The sealer’s effect on cells was assessed by MTT tests. After 24 hours, the cells were washed and incubated with 200 microliters of culture medium along with 50 microliters of MTT solution (2 mg/ml PBS) for 4 hours at 37 °C and away from light. After this period, the above solution was taken out, and 200 microliters of DMSO and 25 microliters of Sorenson glycine buffer (containing glycine and sodium chloride) were added to each well. DMSO dissolved the colored crystals in the mitochondria of the cells, and after transferring the colored solution to the plate, their absorbance was read at 540 nm, and the percentage of living cells was evaluated by comparing the control (cells grown in the absence of the sealer). Cell viability was read as follows:
Cell viability (%) = (OD test- OD blank/OD control- OD blank) ×100
Where, OD test is the absorbance of the new prepared sealers, OD blank is the absorbance of the blank wells (without any sealer or cell), and OD control is the absorbance of wells containing cells grown in the absence of the sealer (Fig. 2).
2.3. Statistical Test
The results of the study have been reported using descriptive statistical methods (mean ± standard deviation). In order to compare the amount of cell death in the three studied groups, one-way ANOVA was used. Statistical analysis was conducted using SPSS version 17 software, and the significance level of p≤0.05 has been considered.
3. RESULTS AND DISCUSSION
Fig. (3) shows the results for cytotoxicity of the new sealer by comparing the control and AH plus groups.
According to ISO 10993-5, which sets the rules for the biocompatibility of medical devices, the acceptable level of cytotoxicity for medical masks is as follows:
If the number of living cells is≥ 80%, the sample is free of cytotoxicity.
If the number of live cells is> 80%, the sample has moderate cytotoxicity.
If the number of living cells is> 40%, the sample has high cytotoxicity [15].
Then, according to Fig. (1), AH plus showed moderate cytotoxic effects against cells (the number of living cells was 49.45 ± 10.56%). However, for the new sealer, the number of living cells was found to be ≥ 80% (81% ± 4.11). Thus, the sample was observed to be non-cytotoxic against the tested cells.
Razavian and his colleagues investigated the cytotoxicity of four types of root canal treatment sealers using human gingival fibroblasts. In that study, all sealers exhibited cytotoxicity. According to their results, the average of all data obtained from sealer, MTA Fillapex (0.78), showed the least cytotoxicity, and ADSEAL (0.60) showed the highest cytotoxicity [12].
In a study, Martins and his colleagues investigated the cytotoxicity of Endo Sealer 6 against endothelial cells at 12, 24, and 72 hours. According to their results, all the investigated commercial sealers showed different degrees of toxicity on the studied cells [13].
Zoufan et al. compared the cytotoxicity of two commercial sealers (GuttaFlow and EndoSequence BC) with AH Plus. Their results showed GuttaFlow and EndoSequence BC sealers to have lower cytotoxicity than the AH Plus. There was also no cell viability difference observed between the EndoSequence BC and GuttaFlow sealer groups in the either freshly mixed or set sealer group [16].
A recent report also presented AH plus to be strictly cytotoxic during the first 3 days and to become noncytotoxic after 3 weeks [17]. These results agree with previous reports that have shown the cytotoxicity to be slowly reduced with time [18]. The higher cytotoxicity, once freshly mixed, may be due to the first release of formaldehyde or the epoxy resin component [19].
CONCLUSION
According to the results of the current study, the new sealer has been found to be non-cytotoxic against the tested cells. However, more investigations are needed on the new sealer in order to validate its biocompatibility and usefulness.
ETHICAL STATEMENT
The ethics code was received from the Ethics Committee of Tabriz University of Medical Sciences, and all steps were performed after obtaining the ethics code (IR.TBZMED.VCR. REC.1401.107).
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The raw/processed data required to reproduce these findings can be shared at this time.
FUNDING
This study has been funded by the Vice-Chancellor for Research (VCR) of Tabriz University of Medical Sciences (Funder ID:TBZMED, Grant number: 69947).
CONFLICT OF INTEREST
Dr. Solmaz Maleki Dizaj is on the editorial advisory board of The Open Dentistry Journal. Dr. Simin Sharifi is on the editorial advisory board of The Open Dentistry Journal.
ACKNOWLEDGEMENTS
This study was based on a thesis (No. 69947) registered at the Faculty of Dentistry, Tabriz University of Medical Sciences, Tabriz, Iran. The authors acknowledge the financial support provided by the Vice-Chancellor for Research at Tabriz University of Medical Sciences, Tabriz, Iran.


