Treatment of Peri-implant Diseases using Lasers: A Systematic Review
Abstract
Background:
Adjuncts to mechanical debridement, including administration of systemically and locally delivered antimicrobials, are constantly evaluated to achieve additional benefits while treating peri-implantitis. However, the potential for the development of antimicrobial resistance limits their use. Evaluation of the use of lasers for the treatment of peri-implantitis has provided varying results.
Objective:
This study aimed to summarize the existing literature on the additional benefits of lasers as adjuncts to mechanical debridement while treating peri-implant diseases.
Methods:
Randomised clinical trials published in English till Sept 2022 in PubMed, Medline, and clinical trial registries using the MeSH terms “peri-implant diseases”, “peri-implant mucositis”, “peri-implantitis”, and “lasers”, were included in the study. Case reports, case series, longitudinal studies, and retrospective analysis were excluded.
Results:
A total of fifteen (n=15) randomised clinical trials pertaining to peri-implantitis and three (n=3) trials pertaining to peri-implant mucositis were assessed. The trials assessed the use of diode, Nd: YAG, Er:YAG, CO2 lasers, and photodynamic therapy as adjuncts in the treatment of peri-implant diseases.
Conclusion:
The existing evidence regarding the use of laser for peri-implant mucositis is inconclusive, whereas for peri-implantitis, the majority of the studies support the use of lasers. Future trials should utilize the 2018 classification of peri-implantitis, so that a comparison of trials based on the studied parameters would be more accurate.
1. INTRODUCTION
Implant-supported prostheses have become a common method for replacing missing teeth [1, 2]. Achieving and subsequently maintaining osseointegration (bone tissue regeneration over the surface of the implant) is important for the stabilization and long-term success of implants [3]. Loss of implants can occur due to several reasons, including periimplant diseases [4]. The term peri-implant diseases encompasses both the terms, peri-implant mucositis and peri-implantitis [4]. Peri-implant mucositis is characterized by erythema and bleeding on the probing of the peri-implant mucosa [4]. This reversible condition is equivalent to gingivitis and may have increased probing depths due to the swelling of the marginal gingiva; however, alveolar bone loss is absent [4]. Contrarily, peri-implantitis is the irreversible inflammation occurring in the peri-implant tissues, caused by subgingival microbial dysbiosis resulting in alveolar bone loss [5, 6]. It is characterized by bleeding on probing, suppuration and/or erythema, deep pockets, and alveolar bone loss [4]. Increased probing depths as detected by a plastic periodontal probe around implants [7] and alveolar bone loss are the characteristic features of peri-implantitis [8]. Mobility of implants may be noticed in advanced stages of peri-implantitis, which may result in the loss of the implant or may necessitate additional surgical procedures resulting in additional expenses for the patient [4, 5].
The prevalence of peri-implant mucositis according to implant and subject-based evaluation was reported to be 29.48% and 46.83%, respectively [9]. Similarly, the prevalence of peri-implantitis according to implant and subject-based evaluation was reported as 9.25% and 19.83% respectively [9]. Another study by Renvert et al. reported the prevalence of peri-implant mucositis and peri-implantitis as 54.7% and 22.1%, respectively [8]. Placing implants into fresh extraction sockets [2, 10], immediate loading of implants [11], implant placement in areas with low bone density (D3 and D4 type of bone) [12], and placement of implants among smokers and uncontrolled diabetics [12], are some of the situations with high rates of peri-implant diseases. Due to the common occurrence of peri-implant diseases and the possible associated complications, these conditions are of high significance. Early identification and ideal intervention for these conditions may prolong the longevity of the implants.
Several treatment options have been explored for the treatment of peri-implantitis. Periodontal debridement mechanically with plastic curettes, saline irrigation, implantoplasty, and access surgery with periodontal debridement, followed by resective or regenerative therapies, are commonly employed [13, 14]. The placement of soft-tissue autografts and allografts has been utilized in the treatment of soft-tissue deficiencies around implants [15, 16]. Several systemic and topical antimicrobials have also been utilized as adjuncts to mechanical therapy while treating peri-implantitis [17, 18]. However, concerns regarding the possible development of antimicrobial resistance with the use of antimicrobials limit their utilization in localized biofilm-related infections. Hence, other treatment options, like lasers (light amplification by stimulated emission of radiation), have been advocated as adjuncts to mechanical therapy [19].
The literature regarding the use of lasers as adjuncts to mechanical therapy for peri-implant diseases has revealed few studies showing improvement in the clinical and microbiological parameters, whereas other studies have shown no additional benefits with lasers being used as an adjunct to mechanical therapy [19, 20]. Different types of lasers, including erbium:yttrium aluminum garnet (Er:YAG), CO2, and diode lasers are utilized as adjuncts to mechanical therapy in the treatment of peri-implant diseases [20]. However, a report summarizing the existing literature regarding peri-implant diseases is currently lacking. Hence, this systematic narrative review aimed to summarize the existing literature regarding laser use in the treatment of peri-implant diseases.
2. METHODOLOGY
A literature search was undertaken using the MeSH terms “peri-implantitis”, “peri-implant mucositis”, and “lasers” in PubMed, Medline, and clinical trial registries up to September 2022. Randomised clinical trials in the English language evaluating the additional benefits (if any) of lasers as adjuncts to mechanical therapy in peri-implant diseases were included. Assessment of probing/pocket depths was considered as inclusion criteria for studies evaluating both peri-implant mucositis and peri-implantitis. As an inclusion criterion, for peri-implant mucositis, a minimal follow-up of three months was essential, whereas for peri-implantitis, a minimal follow-up of six months was needed. Case reports, case series, longitudinal studies, and retrospective analysis were excluded from the review. Translating articles published in languages other than English needs additional time and funding. Hence, research studies published in languages other than English were excluded.
3. RESULTS
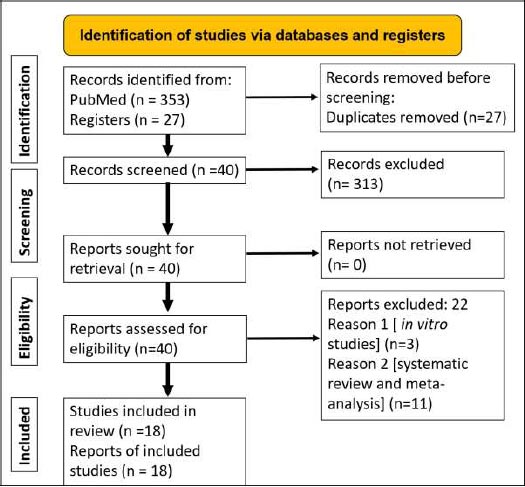
Following the initial search, a total of 353 articles and 27 registrations were identified in PubMed and clinical trial registries (https://clinicaltrials.gov/), respectively. Duplicate records of in vitro studies, systematic reviews and meta-analyses, and best evidence reviews were excluded. The summary of the included and excluded studies and the reasons for exclusion have been included in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart (Fig. 1). A total of 18 randomised clinical trials have been undertaken to assess the additional benefits of lasers as adjuncts to mechanical therapy. Among these trials, the majority of the clinical trials have been on peri-implantitis (n=15) and few trials (n=3) have been carried out on peri-implant mucositis. Among the three trials on peri-implant mucositis, two of the trials did not show additional benefits of using lasers as an adjunct to mechanical therapy. Table 1 summarizes the results of randomised clinical trials evaluating the benefits of lasers as an adjunct treatment to mechanical therapy for peri-implant mucositis. Among the fifteen (n=15) clinical trials conducted on peri-implantitis and lasers, the results of the majority of the clinical trials (n=10) showed additional benefits of using lasers as adjuncts to mechanical therapy. Another five (n=5) trials did not show additional benefits of using lasers as adjuncts to mechanical therapy. Table 2 summarizes the results of randomised clinical trials assessing the benefits of lasers as an adjunct to mechanical debridement for peri-implantitis.
Table 1.
| No. | Author/Year | Follow-up Period | Lasers Assessed | No. of Implants in no. of Patients | Primary and Secondary Outcomes | Changes in Pocket/Probing Depth at Baseline and Follow-up Appointments | Conclusion of the Trial |
| 1 | Tenore et al., 2020 [21] |
3 months |
Diode laser
wavelength: 980 nm; power: 1W; energy density: 14.1 J/cm 2 |
Twenty three (n=23) implants in twenty three (n=23) patients | Bleeding on probing and probing depths | Pocket/probing depth changes in the laser group: 4.04 ± 0.54 mm at baseline 2.98 ± 0.70 mm at 3 months Pocket depth changes in the control group: 3.8 ± 1.24 mm at baseline 3.54 ± 0.35 mm at 3 months |
Significant improvement in probing depths was noticed with the use of diode lasers. |
| 2 | Mariani et al., 2020 [22] |
12 months |
Diode laser wavelength: 980 nm; power: 2.5 W; energy density: 120 J/cm2 |
Seventy three (n=73) implants in seventy three (n=73) patients | Bleeding on probing and probing depth | Pocket depth changes in the laser group: 3.6 ± 0.7 mm at baseline 3.0 ± 0.6 mm at 3 months 3.1 ± 0.7 mm at 12 months; Pocket depth changes in the control group: 3.8 ± 0.6 mm at baseline 3.1 ± 0.4 mm at 3 months 3.3 ± 0.6 mm at 12 months |
Minimal benefits with diode laser used as an adjunct to mechanical debridement. |
| 3 | Aimetti et al., 2019 [23] |
3 months |
Diode laser wavelength: 980 nm; power: 2.5 W; energy density: 120 J/cm2 |
Two hundred and twenty-two (n=222) implants in two hundred and twenty-two (n=222) patients |
Bleeding on probing and pocket depth | Pocket depth changes in the laser group: 3.5 ± 0.7 mm at baseline 3.0 ± 0.5 mm at 1 month 2.9 ± 0.6 mm at 3 months; Pocket depth changes in the control group: 3.4 ± 0.9 mm at baseline 2.9 ± 0.8 mm at 3 months 3.0 ± 0.7 mm at 12 months |
No significant additional benefits with the use of diode laser. |
| No. | Author/Year | Follow-up Period | Lasers Assessed | No. of Implants in no. of Patients | Primary and Secondary Outcomes | Changes in Pocket/Probing Depth at Baseline and Follow-up Appointments | Conclusion of the Trial |
| 1 | Roccuzzo et al., 2022 [24] | 6 months |
Diode laser wavelength: 810 nm; power: 2.5 W; energy density: not mentioned |
Twenty-five (n=25) implants in twenty-five (n=25) patients | Probing pocket depth; clinical, microbiological, and radiographic outcomes. | Pocket depth changes in the laser group: 5.4 ± 0.8 mm at baseline 4.3 ± 0.6 mm at 3 months 4.1 ± 0.8 mm at 6 months; Pocket depth changes in the control group: 5.3 ± 0.5 mm at baseline 3.7 ± 0.6 mm at 3 months 3.8 ± 0.9 mm at 6 months |
No additional benefits with the adjunct use of a diode laser |
| 2 | Yayli et al., 2022 [25] |
6 months |
Diode laser wavelength: 940 nm; power: 0.8 W; energy density: 3J/ cm2 Er,Cr:YSGG laser wavelength: 2780 nm; power: 1.5 W; energy density: not mentioned |
Fifty (n=50) implants in fifty (n=50) patients | Probing pocket depth; TIMP-1, MMP-9, gingival index, bleeding on probing |
Pocket depth changes in the diode laser group: 4.1 ± 0.8 mm at baseline 3.3 ± 1.0 mm at 6 months; Pocket depth changes in the Er,Cr:YSGG group: 4.5 ± 1.1 mm at baseline 3.3 ± 0.9 mm at 6 months; Pocket depth changes in the control group: 4.1 ± 0.6 mm at baseline 3.6 ± 0.7 mm at 6 months |
Er,Cr:YSGG laser was more effective, especially in improving the clinical and molecular parameters |
| 3 | Strauss et al., 2021 [26] | 12 months |
Nd:YAG wavelength: 1064 nm; power: 3.6 W, 20mHz; energy density: 4J/mm |
Thirty six (n=36) implants in twenty (n=20) patients | Probing pocket depth; residual bone levels and bleeding on probing |
Pocket depth changes in the laser group: 6.5 mm at baseline 4.6 mm at 12 months; Pocket depth changes in the control group: 5.3 mm at baseline 4.0 mm at 12 months |
Significant reductions in probing depth and improvement in bone levels following the use of Nd:YAG lasers |
| 4 | Wang et al., 2021 [27] | 6 months |
Er:YAG laser wavelength: 2940 nm; power: not mentioned; energy density: 30 mJ/pulse, 20 pulse per sec |
Twenty four (n=24) implants in twenty four (n=24) patients | Probing pocket depth; clinical attachment level |
Pocket depth changes in the laser group: 7.7 ± 1.9 mm at baseline 6 ± 1.6 mm at 3 months 5.0 ± 1.7 mm at 6 months; Pocket depth changes in the control group 6.4 ± 1.0 mm at baseline 5 ± 0.8 mm at 3 months 4.6 ± 0.7 mm at 6 months |
Er:YAG laser may result in a reduction in probing depths in combination with regenerative periodontal therapy |
| 5 | Almohareb et al., 2020 [28] | 12 months |
Methylene blue mediated using diode laser: photodynamic therapy (PDT) wavelength: 670 nm; power: not mentioned; energy density: not mentioned |
Forty (n=40) implants in forty (n=40) patients | Bleeding on probing, probing depth, clinical attachment level, microbial counts of red-complex bacteria | Pocket depth changes in the laser group: 5.2 ± 2.0 mm at baseline 4.4 ± 1.1 mm at 6 months 3.8 ± 0.9 mm at 12 months; Pocket depth changes in the control group: 5.4 ± 2.1 mm at baseline 4.7 ± 1.0 mm at 6 months 4.1 ± 1.0 mm at 12 months |
PDT was effective in reducing the symptoms of severe peri-implantitis |
| 6 | Alqahtani et al., 2020 [29] | 6 months |
Low-level laser therapy wavelength: 940 nm; power: 0.3W; energy density: 3.41J/ cm2 |
Sixty-seven (n=67) implants in sixty-seven (n=67) patients | Bleeding on probing, probing depth and levels of the crestal bone | Pocket depth changes in the low-level laser group: 5.1 mm at baseline 1.5 mm at 3 months 1.8 mm at 6 months; Pocket depth changes in the control group: 5.2 mm at baseline 3.3 mm at 3 months 4.8 mm at 6 months |
Low-level laser therapy as an adjunct was more effective in reducing bleeding on probing and pocket depths. |
| 7 | Wang et al., 2019 [30] |
6 months |
PDT using toluidine blue wavelength: 635 nm; power: 750 mW; energy density: not mentioned |
One hundred and thirty-one (n=131) implants in one hundred and thirty-one (n=131) patients | Pocket depth and attachment loss | Pocket depth changes in the PDT group: 4.9 ± 1.0 mm at baseline 4.2 ± 0.9 mm at 1 month 3.4 ± 0.4 mm at 3 months 3.0 ± 0.3 mm at 6 months; Pocket depth changes in the control group: 5.0 ± 0.7 mm at baseline 3.5 ± 0.5 mm at 1 month 3.9 ± 0.2 mm at 3 months 4.6 ± 0.4 mm at 6 months |
PDT significantly reduced the pocket depth, gingival bleeding, and clinical attachment loss compared to mechanical debridement alone |
| 8 | Arisan et al., 2015 [31] |
6 months |
Diode laser wavelength: 810 nm; power: 1W; energy density: 3J/ cm2 |
Forty eight (n=48) implants in ten (n=10) patients | Pocket depths and marginal bone loss, microbiology and radiographs | Pocket depth changes in the laser group: 4.7 mm at baseline 4.2 mm at 1 month 4.5 mm at 6 months; Pocket depth changes in the control group: 4.4 mm at baseline 4.0 mm at 1 month 4.2 mm at 6 months |
No additional benefits were observed with the adjunct use of laser. |
| 9 | Papadopoulos et al., 2015 [32] |
6 months |
Diode laser wavelength: 980 nm; power: 0.8W; energy density: not mentioned |
Sixteen (n=16) implants in sixteen (n=16) patients | Pocket depth, clinical attachment level, and bleeding on probing. | Pocket depth changes in the laser group: 5.9 mm at baseline 4.5 mm at 3 months 4.4 mm at 6 months; Pocket depth changes in the control group: 5.5 mm at baseline 4.3 mm at 3 months 4.4 mm at 6 months |
Additional use of diode laser with access surgical therapy did not provide additional benefits. |
| 10 | Bombeccari et al., 2013 [33] | 6 months |
PDT wavelength: 810 nm; power: 1W; energy density: not mentioned |
Forty (n=40) implants in forty (n=40) patients | Bleeding scores, inflammatory exudation, and total anaerobic bacteria | Pocket depth changes in the PDR group: 5.9 ± 0.8 mm at baseline 5.2 ± 1.3 mm at 3 months 4.9 ± 0.5 mm at 6 months; Pocket depth changes in the control group: 5.8 ± 0.8 mm at baseline 5.7 ± 0.5 mm at 3 months 5.5 ± 0.5 mm at 6 months |
Bleeding scores and inflammatory exudates were significantly reduced in the PDT group |
| 11 | Schwarz et al., 2011 [34] |
6 months |
Er:YAG laser wavelength: 2940 nm; power: not mentioned; energy density: 11.4J/cm2, 10 Hz |
Thirty-eight (n=38) implants in thirty-two (n=32) patients | Bleeding on probing and clinical attachment loss | Pocket depth changes in the laser group: 5.1 ± 1.6 mm at baseline 3.4 ± 0.6 mm at 6 months; Pocket depth changes in the control group: 5.5 ± 1.8 mm at baseline 3.1 ± 0.6 mm at 6 months |
The addition of laser therapy to the surgical debridement of peri-implantitis lesions did not provide additional benefits. |
| 12 | Schwarz et al., 2013 [35] |
48 months (four-year follow-up) |
Er:YAG laser wavelength: 2940nm; power: not mentioned; energy density: 11.4J/cm2, 10 Hz |
Twenty one (n=21) implants in seventeen (n=17) patients | Bleeding on probing and clinical attachment loss | Pocket depth changes in the laser group: 5.1 ± 1.6 mm at baseline 3.8 ± 1.1 mm at 48 months; Pocket depth changes in the control group: 5.5 ± 1.8 mm at baseline 4.3 ± 1.2 mm at 48 months |
No additional benefits with Er:YAG laser use as an adjunct to resective/regenerative periodontal therapy |
| 13 | Schwarz et al., 2012 [36] |
24 months |
Er:YAG laser wavelength: 2940nm; power: not mentioned; energy density: 11.4J/cm2, 10 Hz |
Twenty six (n=26) implants in twenty four (n-24) patients | Bleeding on probing and clinical attachment loss | Pocket depth changes in the laser group: 5.1 ± 1.6 mm at baseline 3.2 ± 0.8 mm at 12 months Pocket depth changes in the control group: 5.5 ± 1.8 mm at baseline 3.2 ± 0.4 mm at 12 months |
Use of Er:YAG laser following access surgery was equally effective compared to surface decontamination |
| 14 | Bassetti et al., 2014 [37] |
12 months | PDT wavelength: 660 nm; power: 100 mW for 10 sec; energy density: not mentioned |
Forty (n=40) implants in forty (n=40) patients | Bleeding on probing, pocket depth, clinical attachment level, microbial counts, and crevicular fluid levels | Pocket depth changes in the PDT group: 4.2 ± 0.5 mm at baseline 3.9 ± 0.6 mm at 3 months 3.8 ± 0.6 mm at 6 months 3.9 ± 0.7 mm at 9 months 4. 1 ± 0.8 mm at 12 months; Pocket depth changes in the local drug delivery group: 4.4 ± 0.8 mm at baseline 3.9 ± 0.6 mm at 3 months 3.9 ± 0.8 mm at 6 months 3.9 ± 0.7 mm at 9 months 4.1 ± 0.8 mm at 12 months |
The use of PDT was equally effective in reducing all the clinical parameters compared to the local administration of minocycline microspheres |
| 15 | Persson et al., 2011[38] |
6 months |
Er:YAG laser wavelength: 2940 nm; power: 100mJ, 10Hz; energy density: 12.7 J/cm2 |
Hundred (n=100) implants in forty-two (n=42) patients | The pocket depth and levels of pathogenic periodontal bacteria | Pocket depth changes in the laser group: 6.9 ± 1.4 mm at baseline 5.8 ± 1.5 mm at 6 months; Pocket depth changes in the air abrasive group: 6.5 ± 2.1 mm at baseline 5.2 ± 1.5 mm at 6 months |
Short-term reduction (one month) in the levels of pathogenic bacteria in both the laser group and the air-abrasive group. At 6 months, both the groups showed limited improvement. |
4. DISCUSSION
Among the three randomised clinical trials undertaken on peri-implant mucositis, with lasers as adjuncts to mechanical therapy, two of the trials failed to show significant benefits compared to mechanical debridement alone. Tenore et al. reported significant improvement in probing depths with the use of diode lasers as an adjunct to mechanical debridement of peri-implant mucositis and initial peri-implantitis [21]. This study was followed for three months and the clinical trial had a negative control, which was a positive aspect of the trial [21]. Mariani et al. reported a clinical trial involving 73 peri-implant mucositis patients; the adjunct use of diode laser with mechanical debridement resulted in minimal improvement in the clinical parameters [22]. Though the use of a diode laser may provide benefits in the short term, there have been no significant benefits reported in the long term [22]. In another trial comprising 222 implants, the addition of a diode laser did not significantly reduce bleeding on probing or decrease pocket depths compared to mechanical therapy alone at three months [23]. (Table 2) Hence, with regards to peri-implant mucositis, the addition of lasers to mechanical therapy seems to provide minimal additional benefits (if any) compared to mechanical therapy.
The following section highlights the trials that have shown additional benefits of using lasers as adjuncts to mechanical therapy for peri-implantitis. Almohareb et al. reported similar improvement in the clinical parameters among severe peri-implantitis (with abscess) patients at 6 and 12 months by using both antimicrobial (application of amoxicillin and metronidazole) and photodynamic (methylene blue mediated with diode laser) therapies as adjuncts to mechanical therapy [28]. However, the absence of negative control in the trial design (only mechanical therapy) was one of the limitations of the study [28]. Alqahtani et al. reported significant improvement in bleeding on probing and pocket depths at three and six months with adjunct low-level laser therapy compared to mechanical therapy alone [29]. However, there was no significant improvement in the crestal bone levels in either of the groups at six months [29]. Another clinical trial on peri-implantitis among Chinese Han patients with the use of photodynamic therapy (635 nm laser with toluidine blue) reported improvements in pocket depth, peri-implant plaque index, bleeding index, and clinical attachment level [30] (Table 2).
Bombeccari et al. reported that the use of photodynamic therapy (PDT) resulted in reductions in bleeding index and inflammatory exudates [33]. However, the levels of total anaerobic bacteria were not reduced in the PDT group compared to the surgical therapy alone group [33]. (Table 2) + Bassetti et al. reported the use of PDT as effective as the local application of minocycline microspheres in reducing bleeding on probing, pocket depth, and clinical attachment level [37]. Similarly, the levels of pathogenic bacteria and interleukin-1β in the GCF were reduced in both PDT and minocycline microspheres groups [37].
A few clinical trials have reported no additional benefits in clinical or microbiological parameters following the use of lasers with non-surgical mechanical debridement. Arisan et al. reported no significant differences in pocket depths, counts of periodontal pathogens, and bone levels in laser and control groups among peri-implantitis patients [31]. Papadopoulos et al. reported no significant benefits with diode laser use as an adjunct to surgical therapy [32]. All the clinical parameters, including pocket depth, clinical attachment level, bleeding on probing, and plaque index improved in both the groups [32]. Schwarz et al. reported no additional benefits of adjunct surface decontamination using lasers in patients with peri-implantitis [35]. At two years, the same group of patients showed both the therapies to be equally effective with both laser debridement and manual debridement in terms of reducing bleeding on probing and clinical attachment levels [36].
Persson et al. reported a short-term reduction (1 month) in the levels of periodontal pathogens in both air-abrasive sub-gingival polishing and laser groups [38]. However, by six months, both groups failed to show significant improvements in the clinical parameters [38]. The levels of periodontal pathogens were also not reduced in both groups at six months [38]. Peri-implant diseases are multi-factorial with the accumulation of subgingival plaque in the form of biofilm being the initiating factor [39]. Additionally, biofilms have an inherent ability to be resistant to the action of antimicrobials/biocides [40, 41]. The biofilm complex creates an environment wherein bacterial aggregates adhere to the implant surface and each other with a self-produced matrix of extracellular polymer substance [41]. The bacteria in the biofilm possess various properties, including the production of endotoxins and enzymes capable of destroying the adjacent connective tissue and quorum sensing, which ultimately enhances the virulence of the biofilm [39]. Lasers act by reducing the bacterial load at the implant surface by surface decontamination, and they are also known to possess antimicrobial properties [42]. Photodynamic therapy employs a photosensitizer dye which when activated by the laser light results in oxidative damage causing an anti-microbial effect [43]. Moderately rough surfaces of the dental implants could be another reason for peri-implantitis. Currently, most dental implants possess moderately rough surfaces, which if covered by a biofilm are extremely difficult to clean [44]. Various in vitro studies have been carried out to explore novel surfaces that may have the ability to enhance the attachment of the cells to the implant-abutment framework and simultaneously reduce bacterial adhesion and microbial contamination [45, 46]. Finally, placement of implants without adequate treatment planning and without acquiring optimal training has also been listed as the cause of mishaps during implant procedures and subsequent peri-implantitis [47-49].
4.1. Limitations
This review has a few limitations. Firstly, many of the clinical trials, especially the trials on peri-implantitis, were superior clinical trials, where the efficacy between two types of adjunct therapies (air-abrasive therapy versus laser therapy or adjunct antimicrobial therapy versus laser therapy) was compared. Additionally, many of these trials also lacked a negative control (placebo) in their trial design, which could be considered a limitation of these trials. Secondly, the trials used different types of lasers with varying treatment protocols. Due to the heterogeneity of data concerning the use of the different types of lasers in peri-implant diseases, it is difficult to arrive at definitive conclusions or perform a meta-analysis on the topic. Thirdly, various case definitions have been used for peri-implant diseases, which acts as a deterrent in comparing the different studies. In 2018, the American Academy of Periodontology and the European Federation of Periodontology jointly released a new classification of peri-implant health and peri-implant diseases [50]. Future research on peri-implantitis should follow universally accepted case definitions of peri-implantitis.
4.2. Future Directions
Only a few clinical trials have reported on the adjunct benefits of lasers in peri-implant mucositis. Possible reasons for the lack of studies on peri-implant mucositis and lasers could be due to the difficulty in identifying patients with peri-implant mucositis and the lack of reporting by patients regarding peri-implant mucositis. However, since peri-implant mucositis is reversible, better methods to treat this condition would be beneficial to patients. So, more high-quality clinical trials are needed on the additional benefits of lasers in the treatment of peri-implant mucositis.
CONCLUSION
There is scarce evidence to suggest that lasers as an adjunct to mechanical therapy in peri-implant mucositis provide additional benefits. With regards to peri-implantitis, laser use as an adjunct seems to provide additional benefits, especially with regards to reduction in bleeding on probing, and pocket depths. Although the reduction in the levels of pathogenic bacteria may be short-lived, a few studies report long-term benefits (up to 12 months). Hence, lasers can be used as adjuncts in the treatment of peri-implantitis.
LIST OF ABBREVIATIONS
| LASER | = Light Amplification by Stimulated Emission of Radiation |
| PRISMA | = Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PDT | = Photodynamic Therapy |
| Nd:YAG | = Neodymium-doped Yttrium Aluminum Garnet |
| Er:YAG | = Erbium-doped Yttrium Aluminium Garnet |
| Er,Cr:YSGG | = Erbium, Chromium-doped Yttrium, Scandium, Gallium, and Garnet |
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
This is a systematic review of already published randomised controlled trials on the topic of lasers and peri-implantitis. The data has been retrieved from PubMed database, https://pubmed.ncbi.nlm.nih.gov/, [21-38].
FUNDING
This is a systematic review, and the study did not receive any funds/grants. Article processing fees has been funded by the College of Dentistry, Gulf Medical University, Ajman, UAE.
STANDARDS OF REPORTING
PRISMA guidelines were followed for this study.
CONFLICT OF INTEREST
All the authors declare no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.
SUPPLEMENTARY MATERIAL
PRISMA checklist is available as supplementary material on the publisher’s website along with the published article.


