Bone Augmentation Techniques with Customized Titanium Meshes: A Systematic Review of Randomized Clinical Trials
Abstract
Background:
A correct tridimensional implant placement requires a sufficient amount of bone to completely satisfy the prosthetic reconstruction. Several techniques can be used to recreate the bone quantity. Among them, titanium meshes have shown great potential in space maintenance and fewer complications in case of exposure. Recently, 3D CAD, CAM technology, and specifically SLM have been used to produce customized meshes in titanium alloy. The aim Purpose of this systematic review is to evaluate new customized meshes compared to traditional ones in terms of new volume of generated bone and the incidence of complications.
Materials and Methods:
A MEDLINE/PubMed literature search was performed to find relevant randomized controlled clinical trials published in English up to and including December 2022. The Cochrane Database of Systematic Reviews and SCOPUS were also searched. The main keywords used in the search were: titanium meshe(s), customized titanium meshe(s), combined with AND/OR as Boolean operators, and bone augmentation with/and/or titanium mesh.
Results:
The electronic search identified 1002 papers in total, and after duplicate removal, 500 articles were screened. After a manual screening of the title and abstract, 488 studies were excluded, and 12 articles' full text of 12 articles was analyzed. Further analysis was performed to make sure that the articles matched the inclusion/exclusion criteria of the present review. Six additional articles were excluded in this phase. No meta-analysis was performed due to the heterogeneity of the data.
Conclusion:
By using traditional or customized devices with the newly generated bone volume allowed the implant placement in all cases. Complications were mainly reported as exposure during the healing phase, but the conclusions of whether customized or conventional systems perform one better than the other are still inconclusive.
1. INTRODUCTION
It has been calculated that 8.6 million individuals in the US will suffer from edentulism in the year 2050 [1] and the effect might be higher in developing countries. Persons in the age group 35 to 45 years exhibit, according to WHO guidelines [2],the maximum partial edentulousness prevalence and, because of lack of dental treatment, the condition can rapidly evolve to total edentulousness in older people.
The phenomenon of ooth loss, which can be connected to trauma, periodontal disease, traumatic extractions up to mouth cancer, may lead to moderate to severe bone deficiency. A bone defect is defined as an anatomical condition that doesn’t allow the conventional placement of implants [3]. In order to recreate the lost anatomy, bone augmentation may be required. Much progress was made in the last decades, but still several challenges exist concerning hard tissue augmentation procedures. Bone augmentation procedures are a complex of healing and maturation complex factors because of the biology and physiology of soft and hard tissues, patients’ medical history and risks, the materials employed and the complexity of the surgical techniques [4].
Therefore, conventional grafting materials may show disadvantages because of the brittleness of allografts, xenografts, and alloplasts, not easy porous form generation, and the unpredictable response to generate precise patient-specific structures to match the need for precision medicine. In the meantime, autografts cannot be easily retrieved and shaped for the bony defect demand [5]
Thus, the challenges are exploring novel bone graft substitutes to be used as allografts, xenografts, and alloplasts, or therapies capable of reducing bone resorption and/or supporting bone augmentation.
The basic principle of Guided Bone Regeneration (GBR) consists by placing a mechanical barrier to protect the blood clot and to isolate the bony defect from the connective and epithelial tissue invasion. This space is needed to allow the osteoblasts growth and maturation for bone regeneration. The use of a barrier membrane can have the advantage of facilitating the procedure, but at the same time the shape of the defect itself may create a risk of collapse of the barrier and then the loss of the “space maintaining” effect [6]
Titanium meshes have quite a long history as a predictable technique for bone regeneration and, due to their rigidity, the adaptation to the defect and maintenance of the shape may be more stable [7-10]. To overcome the main drawbacks, like remaining sharp margins after cutting and the increased surgical time needed for their shaping / fitting, pre-shaping of the mesh on a stereolithographic model (STL) of the patient’s jaw can represent an alternative to significantly decrease the intraoperative time, but with a significant cost increase [11]. More recently, 3D-printed, custom-made titanium alloy devices have been introduced as a modern alternative [12].
SLM printing technique works with a laser source that melts the titanium powder alloy following the CAD project [13, 14] and one of the most important aspects related to the obtained product is the post-production process, which may alter or anyway modify the final roughness of the device surface, with important clinical and biological implications [15, 16].
The aim of this systematic review is to evaluate randomized clinical trials focusing on the use of conventional or customized titanium mesh, analysing the most important clinical outcomes, such as the incidence of healing complications and volume of newly regenerated bone.
2. MATERIALS AND METHODS
The present systematic review is reported in accordance with the guidelines for the Transparent Reporting of Systematic Reviews and Meta-analyses [17, 18] following the 2020 Prisma Guidelines [19].
2.1. Focused Questions
The purpose of the review was to analyse, evaluate and compare the reported outcomes of bone augmentation procedure by means of the use of customized devices and conventional non-resorbable titanium meshes.
The focused question was set according to the PICO (population or problem (P), intervention (I), comparison (C), and outcome (O)) strategy as follows:
- Population: Healthy patients with atrophic edentulous maxilla or mandible
- Intervention: Bone augmentation with Titanium meshes
- Comparison: Customized devices and conventional titanium meshes.
- Outcome: Incidence of complications, the total volume of newly generated bone. Incidence of complications included the exposure rate during the healing phase.
2.2. Search Strategy
A MEDLINE/PubMed literature search was performed to find relevant randomized controlled clinical trials published in English up to and including December 2022. The Cochrane Database of Systematic Reviews and SCOPUS were also searched. The main keywords used in the search were: titanium meshe(s), customized titanium meshe(s), combined with AND/OR as Boolean operators and bone augmentations with/and/or titanium mesh. We hand-searched the contents pages of the most relevant journals in the field. In addition, the search was complemented by a manual search of the reference lists of all articles captured.
2.4. Screening Methods
Two reviewers (NDA, CSY) independently performed the primary search and then the screening of the titles and abstracts was done manually. The two reviewers (ZHK,EMY) obtained and independently assessed the full texts of potentially eligible manuscripts to decide and select the studies that met the inclusion criteria. Any discrepancy was resolved through a discussion with a third author (MM).
2.5. Data Extraction
Data were extracted independently by two reviewers (NDA and CSY) using an Excel spreadsheet (Microsoft, Redmond, WA, USA) specifically created for this review. The data extracted included: title, authors, year of publication, focused questions of the study clearly reported, bone augmentation technique mentioned, randomization and allocation concealment, total number of patients reported, and the total number of interventions reported. During this process, any discrepancy was resolved through a consensus discussion with a third author (MM).
3. RESULTS
3.1. Inclusion and Exclusion of Articles
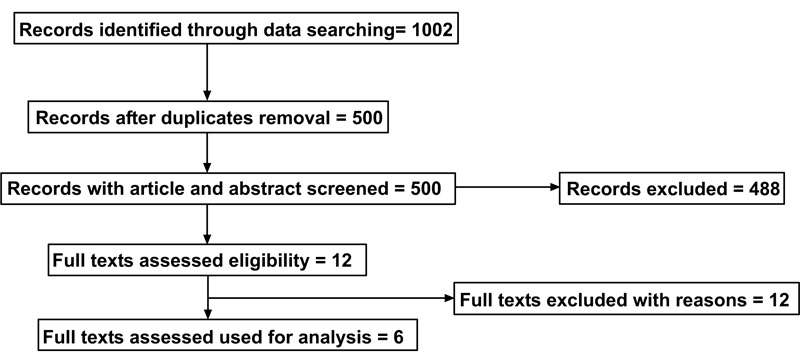
A flow diagram reporting the screening and selection of studies is presented in Fig. (1). The electronic search identified 1002 papers in total, and after a duplicate removal, 500 articles were screened. After a manual screening of the titles and abstracts, 488 studies were excluded, and the full-text of 12 articles was analysed. Further analysis was performed to make sure that the articles matched the inclusion/exclusion criteria of the present review. Six additional articles were excluded in this phase. The reasons for exclusion are shown in Table 1. No meta-analysis was performed due to heterogeneity of the data, evaluated by a groups and subgroups analysis made on each study included in this systematic review.
3.2. Description of the Selected Studies
Six randomized clinical trials from 2010 to 2022 were selected for the analysis. Cucchi A, Vignudelli E, Napolitano A. 2017 [25], Cucchi A, Vignudelli E, Franceschi D. 2021 [26], Cucchi A, Sartori M, Aldini NN. 2019 [27], Cucchi A, Vignudelli E, Fiorino A [28], Torres J, Tamimi F, Alkhraisat MH, Manchón A. 2010 [29], Mounir M, Shalash M, Mounir S. 2019 [30]. Selection and description of the studies are reported in Table 2. Three studies compared the results of bone augmentation with Titanium mesh vs. non resorbable membranes ; one study compared the outcomes of customized titanium meshes vs non resorbable membranes, one study evaluated only the outcomes of the use of Titanium mesh, one study compared the use of the titanium mesh vs. a customized peek mesh. All the included studies reported the statistical analysis methods used for the elaboration of the results, which were evaluated by one independent assessor not included in the list of the authors and expert in the statistical field. (A.A.)
3.3. Quality Assesment of the Studies
Table 3 summarizes the quality assessment of the included studies.
| Author/Refs | Year | Reason of the Exclusion |
|---|---|---|
| Moreno-Egea A, Carrillo-Alcaraz A, Soria-Aledo V [20]. | 2013 | Not dental related |
| Silecchia G, Cavallaro G, Raparelli L, Olmi S, Baldazzi G, Campanile FC [8] | 2015 | Not dental related |
| Koch A, Bringman S, Myrelid P, Smeds S, Kald A [21]. | 2008 | Not dental related |
| Bittner R, Leibl BJ, Kraft B, Schwarz J [22]. | 2011 | Not dental related |
| Schopf S, von Ahnen T, von Ahnen M, Schardey H [23]. | 2011 | Not dental related |
| Eriksen JR [24]. | 2011 | Not dental related |
Table 2.
| Authors/Refs | Year of Publication | Design of the Included Studies | Focused Question | Database | Number of Included Patients | Outcome Evaluation |
|---|---|---|---|---|---|---|
| Cucchi A, Vignudelli E, Napolitano A [25]. | 2017 | Randomized Controlled clinical Trail | Evaluate complications rate and vertical bone gain after Guided Bone Regeneration (GBR) with dense non-resorbable d-PTFE titanium-reinforced membranes versus titanium meshes covered by cross-linked collagen membranes | PubMed | 40 | Complication rate distinguishing between “surgical” and “healing” and between “minor” or “major.”. Primary implants stability and vertical bone gain were also evaluated. |
| Cucchi A, Vignudelli E, Franceschi D [26]. | 2021 | Randomized Controlled Clinical Trial | Patients divided into two groups: Group A: custom-made meshes (Mesh-) and Group B: custom-made meshes with cross-linked collagen membranes (Mesh+) | PubMed | 30 | Surgical/technical and healing complications, “pseudo-periosteum” thickness, bone density, planned bone volume (PBV), regenerated bone volume (RBV), regeneration rate (RR), vertical bone gain (VBG), and implant survival in regenerated areas. |
| Cucchi A, Sartori M, Aldini NN [27]. | 2019 | Randomized Controlled Clinical Trial | 20 patients Ti-reinforced dense polytetrafluoroethylene (d-PTFE) membrane (group A), patients were treated with Ti-mesh and a cross-linked collagen membrane (group B) | PubMed | 40 | Evaluation of the clinical and histologic features and to suggest a classification of this connective tissue after GBR with nonresorbable membranes or titanium (Ti)-mesh plus resorbable membranes |
| Cucchi A, Vignudelli E, Fiorino A [28]. | 2021 | Randomized Controlled Clinical Trial | Patients randomly divided into two groups: reinforced PTFE membranes (group A) and titanium meshes plus collagen membranes (group B). simultaneous implants evaluated after prosthetic restoration at baseline and after 1 year | PubMed | 40 | Clinical and histologic features. A further aim was to suggest a classification of the connective tissue after GBR with non-resorbable membranes or titanium (Ti)-mesh plus resorbable membranes |
| Torres J, Tamimi F, Alkhraisat MH, Manchón A [29]. | 2010 | Randomized Controlled Clinical Trial | Ti-mesh technique using ABB as graft material. In 15 patients, the Ti-meshes were covered with PRP (PRP group), whereas in the other 15 the Ti-meshes were not covered with PRP (control group) | PubMed | 30 | Complications and bone formation (clinical, radiographic, and histological evaluation) |
| Mounir M, Shalash M, Mounir S [30]. | 2019 | Randomized Controlled Clinical Trial | Three dimensional (3D) maxillary ridge augmentation: Pre bent Titanium meshes and Customized peek mesh | PubMed | 16 | Assessment included measurements of linear changes in the vertical and horizontal dimensions on cross sectional cuts of cone beam computed tomography using special software. Finally; the percentage of 3D bone gain in each group was compared to that of the other. |
3.4. Bone Augmentation with Titanium Mesh
Many case reports and pilot studies describe the use of Titanium meshes, both conventional and customized. All studies included in this review used this technique to achieve the desired bone volume. Two of them [26, 30] report the use of customized devices, while the other four compare the outcomes of conventional prefabricated meshes alone versus nonresorbable PTFE membranes.
| Criteria | Cucchi A, Vignudelli E, Napolitano A. [25] | Cucchi A, Vignudelli E, Franceschi D. [26] | Cucchi A, Sartori M, Aldini NN. [27 | ]Cucchi A, Vignudelli E, Fiorino A. [28] | Torres J, Tamimi F, Alkhraisat MH, Manchón A. [29] | Mounir M, Shalash M, Mounir S. [30] |
|---|---|---|---|---|---|---|
| 1. Research question and inclusion criteria | - | - | - | - | - | - |
| 2. Protocol registered before commencement of the review | - | - | - | - | - | - |
| 3. Explanation of randomization process and allocation concealment | - | - | - | - | - | - |
| 4. Adequacy of the literature search | - | - | - | - | - | - |
| 5. Risk of bias | - | - | - | - | - | - |
| 6. Sources of financing | - | - | - | - | - | - |
| 7. Appropriateness of statistical evaluation | - | - | - | - | - | - |
| 8. Conflicts of interest | - | - | - | - | - | - |
| Author/Refs | Surgical Complications | Healing Complications | Treatment Proposed |
|---|---|---|---|
| Cucchi A. et al. [25] | Four surgical complications occurred in four different patients. All complications belonged to Class B | 7 complications in 39 patients during the healing period . PTFe group: three complications, overall complication rate of 15.0% ; Ti mesh group: four complications, overall complication rate of 21.1% | Not reported |
| Torres J. et al [29]. | Not reported | In the control group, 28.5% of the cases mesh exposure, while in the PRP group, no exposures registered. | Coverage of the mesh with autogenous PRP |
| Cucchi A. et al [26] | No failures or damages by moving the surgical flaps over the mesh were observed; moreover, no vascular or flap lesions; four neurological lesions were reported | Three early and two late ex- posure of the meshes for a total of 5 (two class 2; three class 3), and two infections without exposure (two class 4) | Early exposed meshes were removed from one to three months after surgery; in cases of late exposure or infections, the meshes were removed within seven days after the complications., Implants always inserted but in two cases an adjunctive GBR procedure was required. |
| Mounir M. et al. [30] | Not reported | In one patient in each group were the meshes were exposed 2 weeks' postsurgery. | Not reported |
| Authors/Refs | Bone Regenerated Volume | Successful Implants Placement |
|---|---|---|
| Cucchi A, et al. [25]. | Vertical bone gain (VBG) in the two groups: Group A, the VBG was 4.2 +/- 1.0 (range, 2.7–5.8) mm ; in Group B, VBG was 4.1 +/- 1.0 (range, 2.6–6.3) mm. | Yes in both groups |
| Cucchi A, et al. [26] | Group Mesh- showed values of 1019.33 mm3, 216.27 mm3, and 803.07 mm3 for PBV, LBV, and RBV, respectively; in the group Mesh+, values of 1022.0 mm3, 178.87 mm3, and 843.13 mm3 were measured for PBV, LBV, and RBV, respectively. | Yes in both groups but two cases that required an adjunctive GBR procedure |
| Cucchi A, et al. [27] | The vertical bone gain was 4.2 ± 1.0 mm in Group A and 4.1 ± 1.0 mm in Group B. Group A had a higher bone density and greater amounts of type 1 periosteum than Group B (P = .01 for both) | Yes in both groups |
| Cucchi A, et al. [28] | Implants showed change in PBL from 0.12 to 0.76 mm, with marginal bone loss of 0.67 and 0.61 mm for group A and group B, respectively. No statistical differences | Implants placed simoultaneously with GBR procedure |
| Torres J, et al. [29] | Bone augmentation was higher in the PRP group than in the control group. | Yes in both groups |
| Mounir M, et al. [30] | Reported volume gain in both groups | Yes in both groups |
3.5. Incidence of complications
Four of the included studies [25, 26, 29, 30] report complications during the healing phase. Details are described in Table 4, divided in surgical complications (occurred during the intervention) and healing complications, after the placement of the device, including exposure and infection, as proposed by Fontana at al. in 2011 [31]. None of the included trials compared the incidence of complications between customized and non customized devices.
3.6. Total Volume of Newly Generated Bone
All the studies included in the systematic review report data about the regenrated bone volume, which are described in Table 5.
4. DISCUSSION
4.1. Bone Volume Gain
Data retrieved by the analysed studies report an average successful outcomes for GBR procedures with titanium meshes and nonresorbable membranes with and without titanium reinforcement. The newly generated bone volume is always sufficient for a correct final implant placement, although in one of the studies [26] by Cucchi et al. two cases required an additional grafting procedure at the time of the implant placement.
The selected RCT compare or the use of titanium conventional meshes with and without coverage with resorbable membranes or the use of customized titanium devices, but no comparisons are made in the same study between the two different types of meshes.
Despite this last consideration, three different articles [25, 26, 28] report an average of vertical bone gain comparable between customized and non customized titanium meshes. These studies have been conducted by the same group of operators and, although the authors declared a randomization process of the treatments, the risk of bias seems quite relevant and it remains unclear when the choice of a customized device gives additional benefits to the procedure. None of the included studies report the different thickness between conventional and SLM fabricated titanium meshes, which might affect the final volume of regenerated bone, but mostly the chemical composition of the alloy used for the fabrication of Grade 4 customized meshes is different from the non customized ones, classified as Grade 5 [16].
Torres J. et al. [28] report the use of autogenous PRP to cover the titanium mesh and the final volume of bone seems to be higher in the group where the platelet derived membrane was employed. Beside the better protection of the graft and the wound, this results might be also related to a direct biological stimulation due to the increased amount of local growth factors [4, 10], but it doesn’t clarify whether the choice of the barrier is the real benefit to the procedure.
Mounir M. et al. [29] introduced polyethilenchetone (PEEK) as an alternative material for the fabrication of customized devices, but neither in the final bone volume, nor in the healing phase statistical differences where noticed between the two materials. One possible benefit could derive from the cost of the raw material and the consequent faster and simpler 3D printing process of a plastic material versus a metal alloy [32].
Titanium mesh shows excellent mechanical properties, its high strength and stiffness enable space maintenance and support for osteogenesis, its stability is mandatory to maintain bone graft volume during the healing, and the elasticity can reduce the oppression of oral mucosa [15]. Due to its plasticity, titanium mesh can be adapted to different bone defects through bending and shaping. These features allow GBR with titanium mesh to show a high stable osteogenesis effect, and achieve instantaneous bone augmentation in horizontal and vertical directions [33].
Therefore the costs - benefits ratio seems to be directed more on the titanium rather than other non resorbable polymers for customized devices, since the second surgery is still needed for the device removal.
An interesting concept has been introduced by Cucchi et al. 2019 [26] related to the pseudo-periostium classification. In essence, it consists on the quantification of the layer of connective tissue,that can be observed above the newly formed bone. In this study, the authors aim to evaluate the clinical and histologic features and to suggest a classification of this connective tissue after GBR with nonresorbable membrane (Group A) or titanium (Ti)-mesh plus resorbable membranes (Group B). Pseudo-periosteum was classified into Type 1 (no tissue or tissue < 1 mm); Type 2 (regular tissue between 1 and 2 mm); and Type 3 (irregular tissue or tissue > 2 mm). Results showed that the vertical bone gain was 4.2 ± 1.0 mm in Group A and 4.1 ± 1.0 mm in Group B. Group A had a higher bone density and greater amounts of type 1 periosteum than Group B (P = .01 for both). The results of this study show that both d-PTFE membranes and Ti-mesh plus collagen membranes are two valid options for bone augmentation in the mandible. However, nonresorbable membranes achieve higher bone density and a thinner pseudo-periosteum layer above the newly formed bone, which, in other words, can favor the total amount of newly generated bone.
4.2. Incidence of Complications
All the included studies evaluated the incidence of complications, which, for a better comprehension of the reader, have been analysed following the classification proposed by Fontana et al. in 2011 [31].
Basically, there are two types of complications: surgical, which may happen during the intervention, such as flap ruptures, damage to anatomical structures, and complications which occur during the healing phase, mostly related to the exposure of the barrier. Moreover, these latter events can be furtherdivided into four classes, according to
the presence and dimension of exposure, as well as the presence of a purulent exudate.
- Class I: Membrane exposure < 3 mm, no purulent exudate.
- Class II: Membrane exposure > 3 mm, no purulent exudate.
- ClassIII: Membrane exposure, with purulent exudate.
- ClassIV: Abscess, without membrane exposure.
Healing complications can be also divided into major or minor, depending on the influence on the regenerative process for newly formed bone . Two studies Cucchi et al. 2017 and Cucchi et al. 2021 [24, 25] follow these criteria; the study conducted in 2017 reports four surgical complications and seven healing complications. Surgical complications were related to neurological paresthesias but no vascular or flap damages. Out of the seven healing complications, two cases in the group treated with PTFE membranes affected the amount of new bone or the success of the bone augmentation surgery and they were, therefore, classified as major complications. In the group treated with Titanium meshes, four cases of healing complications were observed, leading to an overall complication rate of 21.1% . Of these, three (Class III and IV) were classified as major complications and one (Class II) was classified as a minor complication. No statistically significant difference was observed between the two groups regarding the healing complication rates (P = .69) or the major or minor healing complication rate (P = .99).
In the other study [25] Surgical/technical complication rates were 13.3% and 26.7% for the analysed groups, respectively, and no statistically significant differences were observed (p-value = .65). Regarding healing complications, three early and two late exposures of the meshes occurred for a total of 5 (two class 2; three class 3), and two infections without exposure (two class 4) were observed during the healing time. In these seven cases, complications were managed as follows: early exposed meshes were removed from one to three months after surgery; in cases of late exposure or infection, the meshes were removed within seven days after the complications were observed. In all cases, implants were placed as planned, but in two cases an adjunctive GBR procedure was required.
The other studies included in this review do not report a detailed classification of the complications and generally attribute unfavourable events to exposure, which, eventually, did not jeopardize the final implant placement.
These results are in accordance with a previously published systematic review [9] on the use of titanium meshes for bone augmentations, where three outcome variables were defined: a) horizontal and vertical bone regeneration, b) complication rate, defined as the percentage of membrane exposures and c) evaluation of implant survival, success and failure rate. The final results were comparable with those reported in case of bone regeneration obtained through other types of non-resorbable membranes. An advantage in favour of the titanium mesh was found in terms of bone loss after exposure, as implant placement was not jeopardized in almost all of the cases.
One of the advantages for customized devices is the precision and the better fitting onto the defect, therefore it can be expected that surgical complications are less than those where conventional barriers are employed. However, it doesn’t seem from the analysed studies, that healing complications are reduced with the use of customized titanium meshes. In a retrospective study published in 2020 [34] the aim was to evaluate a new protocol for customized bone augmentation in a digital workflow. Patients and augmentation sites were retrospectively analysed based on defect regions, demographic factors, healing difficulties and potential risk factors. In 25% of the cases, exposures of the meshes were documented. Within this exposure rate, most of them were slight and only punctual (A = 16.2%), like one tooth width (B = 1.5%) and completely (C = 7.4%).
Data extracted from the included studies and those reported from the above -mentioned one do not show a lesser exposure rate of the customized meshes when compared to conventional ones.
One of the possible explanations can be related to two factors: the alloy used for the fabrication of customized devices and the thickness.
The titanium alloy used for the production of the meshes is Grade 4, which has a different composition compared to the Grade 5 alloys, used for customized devices; the unexpected presence of carbon retrieved in two studies [15, 16] demonstrates a different chemical composition of the alloy and can be related to a different host response.
Another important aspect is the thickness; infact traditional meshes are produced with an average thickness of 0,3mm, while customized devices are thicker (from 0,5mm to 0,8mm according to the manufacturer). As discussed above, the layer of soft tissues used for the surgical closure is extremely thin and represented only by the epithelial part, thus an unexpected trauma or simply a sudden movement of the lip or the chin may generate tension on the flap, which can easily relapse.
CONCLUSION
Bone regeneration has been widely exploited and has become quite a routine procedure, but there are still some aspects that need further investigation. Surely, the digital workflows and CAD/CAM technology were able to augment the precision, implement the fitting and probably reduce the surgical time, but as a needed clarification, randomized clinical trials with comparison between customized and non customized titanium meshes should be advocated in order to guide the operators to the best options.
Besides the reasons of possible exposure and although the final bone volume after grafting can be fully or partially achieved with the majority of the techniques, the problem related to the second surgery remains the biggest issue when titanium meshes are used, because of an increased risk of complications and social costs.
For a promising future and as a consequence of increased social and operative costs, combined with the tendency of metal- free solutions, technology is exploiting and investigating polymeric materials, which immediately may bring several advantages, such as complete resorption – no need for second surgery and the absence or minimal risk of host pollutions with metal parts during the removal.
LIST OF ABBREVIATIONS
| GBR | = Guided Bone Regeneration |
| STL | = Stereolithographic Model |
| PEEK | = polyethilenchetone |
| GBR | = Guided Bone Regeneration |
AUTHORS’ CONTRIBUTION
Conceptualisation and manuscript preparation were carried out by NDA. Data collection, analysis ZHK and EMY, paper writing CSY. Overall supervision and proofreading MM.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIAL
The data supporting the findings of the article is available in the IRIS SYSTEM University of Genoa archives at https://iris.unige.it/mydspace.
STANDARDS OF REPORTING
PRISMA guidelines were followed.
FUNDING
None.
CONFLICT OF INTEREST
All authors report no conflict of interest, financial or otherwise.
ACKNOWLEDGMENTS
Declared none.
SUPPLEMENTARY MATERIAL
PRISMA checklist is available as supplementary material on the publisher’s website along with the published article.


