All published articles of this journal are available on ScienceDirect.
Clinical and Radiographic Evaluation of Combined Acemannan and Periodontal Surgery Induced-Periodontal Regeneration: 5-Year Follow-up Case Report
Abstract
Background and Objective:
Acemannan, a polysaccharide extracted from aloe vera gel, accelerates oral wound healing, reparative dentin formation, and bone formation in extraction sockets. In this report, we evaluated the efficacy of combined acemannan and periodontal surgery on periodontal regeneration in severe periodontitis cases requiring periodontal surgery.
Case Presentation and Discussion:
Three chronic periodontitis patients with probing pocket depths of at least 6 mm and two- or three-walled vertical bone defects after initial periodontal therapy were included. The patients underwent minimally invasive periodontal surgery with acemannan sponges placed in the defect. Maintenance recall was scheduled every 6 months for 5 years. Clinical and radiographic evaluations were performed to assess the effects of the combined acemannan and periodontal surgery on periodontal regeneration. The patients demonstrated improved clinical parameters and increased radiographic bone fill at the 5-year follow-ups. The percentage bone fill in the three-walled defect, combined two- and three-walled defect, and two-walled defect cases were 70%, 60%, and 20%, respectively. The underlying mechanism of action of acemannan in periodontal regeneration was also discussed.
Conclusion:
Acemannan sponges can be used as an adjunct to periodontal surgery for periodontal regeneration.
1. INTRODUCTION
Periodontitis is a common chronic infectious disease with a high prevalence in middle-aged and elderly people. The disease chronically and progressively damages the periodontal tissues supporting the teeth [1]. Although conventional treatment by scaling, root planing, and tooth polishing can remove the infected tissues and calculus, thereby stopping disease progression, incomplete regeneration of the damaged periodontal tissue is typically observed in patients with severe periodontitis [2]. Biomaterials have been demonstrated to induce periodontal regeneration [3]. However, the high cost of regenerative materials and procedures place an economic restriction on patients’ ability to obtain appropriate treatment.
Acemannan, the major polysaccharide extracted from aloe vera gel, has been shown to stimulate oral wound healing, reparative dentin formation, bone formation in extraction sockets, sinus augmentation, as well as preventing local osteitis [4-7]. Chantarawaratit et al. reported acemannan induced periodontium regeneration in a canine class II furcation defect model of intact periodontal tissue [8]. However, the effect of combined acemannan and periodontal surgery on new periodontium formation in patients with severe periodontitis with an infrabony defect is not known. Here, we report the efficacy of the combined acemannan and periodontal surgery on periodontium regeneration in 3 severe periodontitis cases requiring periodontal surgery after 5 years of follow-up.
2. CASE REPORTS
2.1. Case #1
A 51-year-old man came to the Periodontal Department, Faculty of Dentistry, Naresuan University with a chief complaint of tooth mobility of the left mandibular second molar. There were no abnormalities on extraoral examination and no history of systemic disease or smoking. On intraoral examination, tooth 37 had grade 1 mobility with a 9-mm distobuccal pocket and 10-mm clinical attachment loss. The periapical radiograph revealed a distal vertical bone defect. Based on the clinical and radiographic examinations, this patient was diagnosed with chronic periodontitis.
In this case, periodontal treatment was performed using non-surgical and surgical procedures. Non-surgical treatment was provided using scaling and root planning. The patient was evaluated 1 month later, at which time periodontal surgery was indicated. The operator explained the treatment procedures, and the patient provided informed consent for therapy and publication. Acemannan sponges were prepared by dissolving 20 mg acemannan in 0.2 ml sterile distilled water, frozen at −80°C overnight, and then lyophilized for 24 h. The sponges were sterilized by gamma irradiation (Thailand Institute of Nuclear Technology, Bangkok, Thailand). After creating an intrasulcular incision, full-thickness flaps were elevated. The granulation tissue and remaining calculus were thoroughly removed by curettage. Based on the number of residual bone walls [9], the infrabony defect was classified as a three-wall defect. The defect was filled with an acemannan sponge. The flaps were repositioned and sutured with 4.0 nonabsorbable sutures.
Postoperatively, the patient was prescribed 0.12% chlorhexidine solution and ibuprofen 400 mg (twice per day for 3 days, as needed) [10]. The patient was called to assess any postoperative complications on days 1 and 3. An appointment was made 14 days postoperatively for suture removal. The patient was instructed to maintain his oral hygiene and received professional prophylaxis at 1, 3, and 6 months after surgery. Maintenance recall was scheduled every 6 months.
Digital periapical radiographs were taken immediately (baseline), 6-, 12-months, and 5-years after periodontal surgery using standard parameters (70 kVp, 7 mA, and 0.2-s exposure time). Uniweb Software version 8.1 (Carestream Health, Inc., Rochester, NY) was used for measuring the alveolar bone height. Points A, B, C, and D in Fig. (1) represent the cementoenamel junction (CEJ), alveolar bone crest, the bottom of the bone defect, and apex, respectively. The examiners measured the tooth axis height between Points A and C (AC), Points A and D (AD), and Point B and C (BC). The percentage defect fill was calculated based on Kitamura et al. [11]:
% defect fillt = 100 × [ACbaseline − (AC t × ADbaseline/ADt)]/ (BCbaseline),
where t = 6-months, 12-months, and 5-years post-treatment.
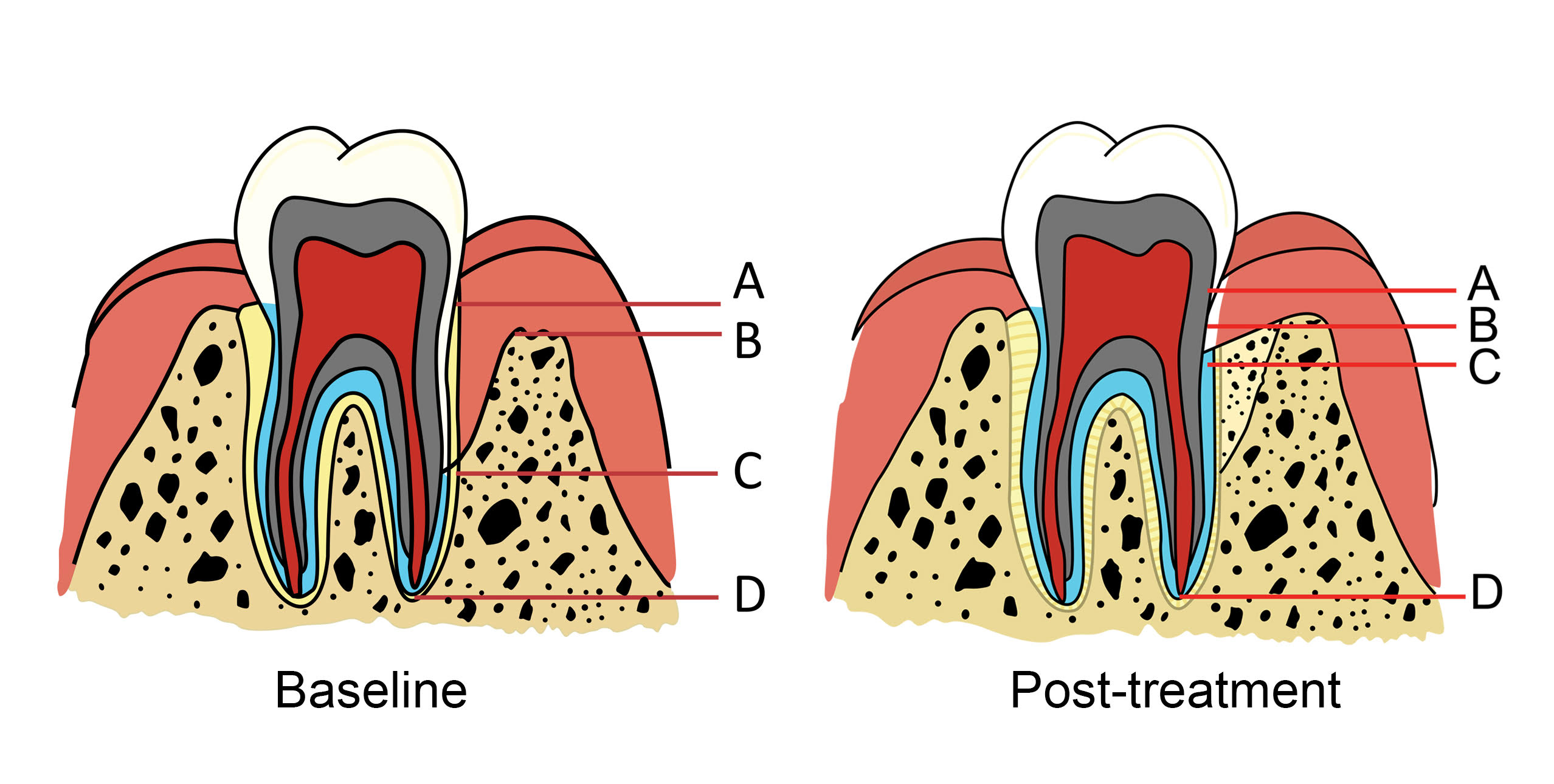
The clinical and radiographic parameters were evaluated by two expert periodontists and an oral radiologist, who were blinded to the treatments. The parameters were reexamined by the same investigators 2 weeks after the initial evaluation. The intra- and inter-rater kappa values were 0.94 and 0.85, respectively.
The patient received radiographic and intraoral examinations at 6-, 12-months, and 5-years post-surgery. An improved periodontal status was observed compared with the baseline (Fig. 2 and Table 1). At the 5-year follow-up, the clinical examination demonstrated a marked improvement where the distobuccal pocket depth had reduced from 9 to 4 mm, 5-mm clinical attachment, and no mobility was found. The radiographic periapical appearance demonstrated marked bone formation characterized by decreased radiolucency in the infrabony defect. The percentage of bone fill was 70%.
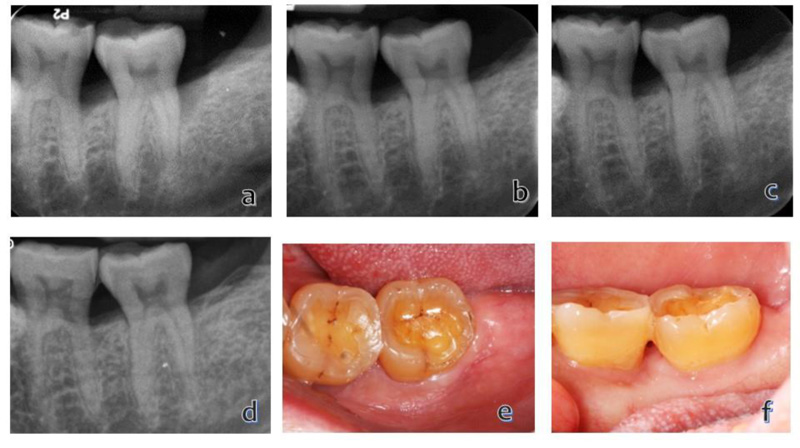
Table 1.
| Subject No. | Clinical Attachment Level (mm) (% gain) | Periodontal Probing Depth (mm) (% reduction) | % Defect Fill | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6 m | 12 m | 5 y | Baseline | 6 m | 12 m | 5 y | 6 m | 12 m | 5 y | |
| 1 | 10 | 5 (50) | 5 (50) | 5 (50) |
9 | 5 (44.4) | 4 (55.6) | 4 (55.6) |
65.5 | 68.9 | 70.2 |
| 2 | 7 | 6 (14.3) | 6 (14.3) | 4 (42.9) | 6 | 5 (16.6) | 5 (16.6) | 4 (33.3) |
34.2 | 55.9 | 59.5 |
| 3 | 8 | 5 (37.5) | 5 (37.5) | 5 (37.5) | 7 | 3 (57.1) | 3 (57.1) | 2 (71.4) |
15.9 | 16.1 | 21.4 |
2.2. Case #2
A 51-year-old woman visited the Dental Hospital, Faculty of Dentistry at Naresuan University with a chief complaint of gingival swelling at the right mandibular second molar. There were no abnormalities on extraoral examination and no history of systemic disease or smoking. On intraoral examination, grade 1 mobility was found on tooth 47 with a 6-mm distobuccal pocket and 7-mm clinical attachment loss. The periapical radiographic revealed a distal bony defect. Based on her clinical and radiographic examinations, this patient was diagnosed with chronic periodontitis.
Periodontal treatment was provided using the aforementioned non-surgical, surgical, and post-surgical procedures. Informed consent was obtained from the patient for treatment and publication before surgery. In this case, the infrabony defect was defined as a combination of a three-wall component in the most apical portion and two-wall component in the more superficial portion of the defect
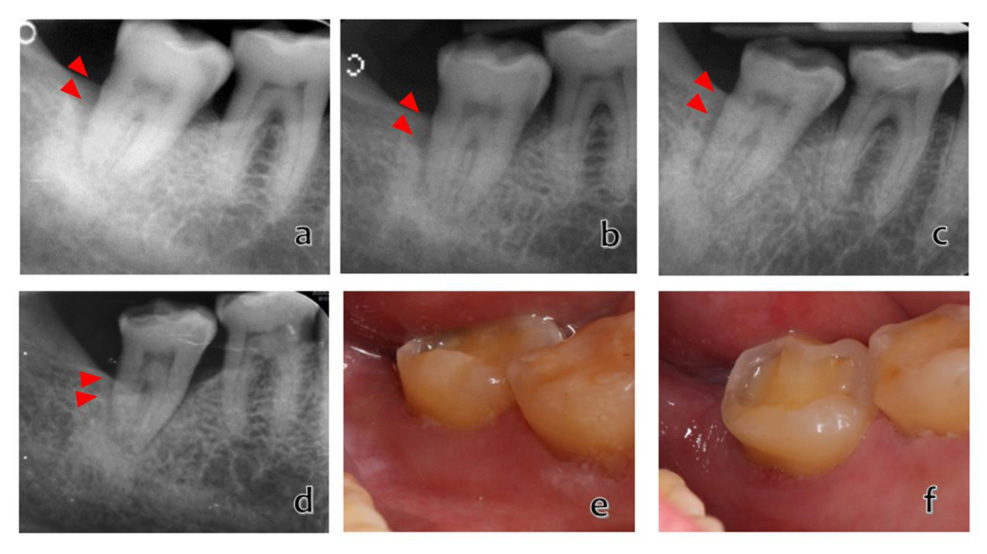
Based on the clinical and radiographic observations, the patient demonstrated an improved periodontal condition compared with the baseline (Fig. 3 and Table 1). At 5-years post-treatment, she demonstrated a 33% reduction in distobuccal pocket depth, a 42.9% clinical attachment gain, and no mobility was detected. The radiographic periapical appearance had markedly increased bone formation characterized by increased radiopacity in the infrabony defect. The bone fill percentage was ~60%.
2.3. Case #3
A 57-year-old man visited a periodontist with the chief complaint of tooth mobility of the left mandibular first premolar. There were no abnormalities on extraoral examination and no history of systemic disease or smoking. On intraoral examination, grade 2 mobility was found on tooth 34 with a 7-mm distobuccal pocket and an 8-mm clinical attachment loss. The periapical radiograph presented a distal infrabony defect. Based on his clinical and radiographic examinations, this patient was diagnosed with chronic periodontitis.
Periodontal treatment was provided using the aforementioned non-surgical, surgical, and post-surgical procedures. The operator explained the treatment procedures, and the patient gave informed consent for treatment and publication prior to surgery. The intrabony defect was classified based on the number of residual bone walls as a two-wall defect.
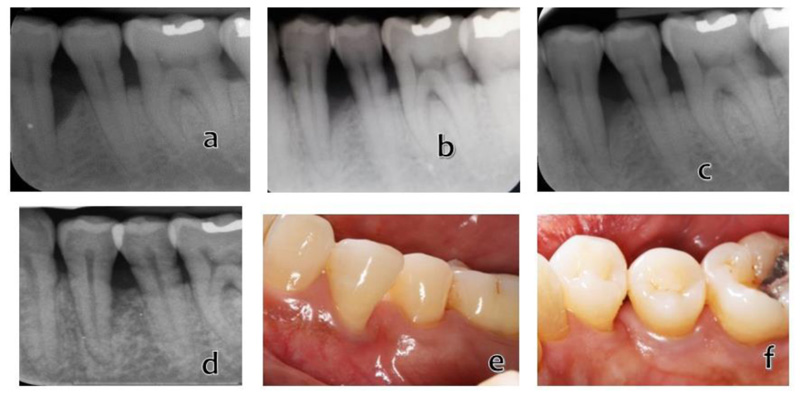
Consistent with the results obtained in the previous patients, there was a meaningful improvement in the clinical results at the follow-up time points (Fig. 4 and Table 1). At 5-years post-surgery, the clinical data revealed a marked decrease in distobuccal pocket depth, i.e., from 7 to 2 mm with 2-mm recession, a 3-mm gain in clinical attachment, and the mobility grade was reduced to grade 1. The periapical radiographic appearance demonstrated bone formation characterized by decreased radiolucency in the infrabony defects. A 20% bone fill was observed.
3. DISCUSSION
In this report, sponges composed of acemannan, a polysaccharide extracted from aloe vera gel, were used as a biomaterial in periodontal surgery to investigate its effects on periodontium regeneration. We observed increased alveolar bone formation, periodontal ligament reattachment, and reduced infrabony pocket depth in the combined acemannan and periodontal surgery treatment up to 5 years post-treatment. These findings corresponded to previous in vivo reports that acemannan stimulated periodontal regeneration in canine class II furcation defects [8].
The percentage defect gain in the teeth that received the combined treatment was lower than that reported by Chantarawaratit et al. These defects may have been caused by chronic pathogen-induced periodontitis. This may be because the surrounding periodontal ligament, cementum, and alveolar bone experienced long-term exposure to bacteria and the subsequent chronic immune response that is responsible for tissue destruction, not an experimental defect created in an intact periodontium. In addition, our patients were 51–57 years old, ages at which cellular and tissue responses and healing rate are reduced. We observed that treating the three-walled defect (patient #1) with an acemannan sponge resulted in better outcomes compared with the combined two- and three-walled and two-walled defects (patient #2 and 3). This finding may be explained by the enriched blood supply from the surrounding bone and increased graft/blood clot retention [12].
Given the limitations of this case report, we were not able to explain the underlying mechanism of action of acemannan in periodontal regeneration. In both in vitro and in vivo studies, acemannan has been shown to function as a bioactive molecule that stimulates periodontal ligament cell proliferation, extracellular matrix (type I collagen, osteopontin, and bone sialoprotein) synthesis, growth factor (BMP-2, BMP-4, GDF-5, and VEGF) secretion, and mineral deposition and to activate transcription factors (Runx2 and NF-κB). Acemannan also modulates the immune response by upregulating IL-6 and IL-8 expression, which play important roles in inflammation and periodontal regeneration [13-15]. The periodontal ligament is crucial for periodontal regeneration. This tissue contains stem cells that proliferate and differentiate into the periodontal ligament cells, cementoblasts, and osteoblasts responsible for generating the periodontal ligament, cementum, and alveolar bone, respectively [16-18]. Moreover, acemannan sponges function as temporary scaffolds for cell recruitment, cell attachment, and tissue formation [8].
Clinical attachment level, periodontal pocket depth, and radiographic alveolar bone height are commonly used as parameters of success in periodontal treatment [18]. Increased treatment success is reflected by increased periodontal ligament reattachment and alveolar bone formation, and reduced periodontal pocket depth. However, these clinical parameters are insufficient to evaluate the effectiveness of a biomaterial in tissue regeneration at the cellular and tissue levels. A 5-year follow-up has been used as an alternative to histopathological evaluation to determine successful clinical treatment outcomes. We found that the combined acemannan and periodontal surgery demonstrated positive outcomes in periodontal pocket depth, clinical attachment gain and radiographic alveolar bone height at the 6-, 12-month, and 5-year follow-ups. These data confirmed the effects of the combined acemannan and periodontal surgery in regenerating the periodontium.
CONCLUSION
The classic literature review by Laurell [19] found that open flap debridement surgery provided limited pocket depth reduction, i.e., 1.5 mm clinical attachment level gain, and 25% percentage bone fill, whereas periodontal surgery with a bone graft or with guide tissue regeneration (GTR) demonstrated better outcomes, with an average clinical attachment level gain of 2.5–4.2 mm and bone fill of 55–57%. In our case report, periodontal surgery with acemannan sponges resulted in a reduced probing pocket depth, increased clinical attachment level of 3–5 mm, and 70% bone fill rate of the initial defect, reflecting a better outcome than that with open flap debridement alone and close to the outcome of periodontal surgery with a bone graft or GTR [20]. A study comparing using GTR, GTR with a bone graft, and GTR with acemannan is currently underway.
It should be noted that the improvement in periodontal parameters we observed could be due to calculus and infected periodontal tissue removal, the inductive effect of the acemannan sponge, or the synergy of both effects. Based on the limited data, our results suggest that the combined acemannan and periodontal surgery enhanced periodontal regeneration over a 5-year period. Acemannan sponges could be a useful adjunctive plant-derived biomaterial with periodontal surgery for periodontal regeneration.
AUTHOR’S CONTRIBUTIONS
We thank Associate Professor Dr. Weeraya Tantanapornkul and Dr.Chutamas Deepho for their valuable suggestions to improve the manuscript. We also thank Dr. Kevin A. Tompkins for revising the language in the manuscript.
ABBREVIATION
| CEJ | = Cementoenamel Junction |
CONSENT FOR PUBLICATION
Informed consent was obtained from the patients for treatment and publication.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the corresponding author, [P. T], on special request.
FUNDING
This work was supported by the Ratchadaphiseksomphot Endowment Fund of the 90th Anniversary of Chulalongkorn University Fund (CU_GR_63_08_32_01) and Thailand Government Research Fund (GB-A_60_004_32_01), and the 100th Anniversary Chulalongkorn University Fund for Doctoral Scholarship.
ACKNOWLEDGEMENTS
Declared none.


