All published articles of this journal are available on ScienceDirect.
Histopathological Findings of Oral Mucosa in Smokeless Tobacco Users: Case Report
Abstract
Background:
New tobacco products, such as smokeless tobacco, are becoming more popular every year. In talking with our patients, we determined several reasons for that trend. The sale of these products is prohibited in many countries; hence, people obtain the product illegally. This is important, since when these products are stored under inappropriate conditions and temperatures, the quality and properties of the product change, including their carcinogenic properties. Sometimes people use a lot of this product or more than one tobacco product daily. It is challenging for dental practitioners to question their patients about tobacco consumption and more challenging to visually detect oral mucosal changes, because patients usually do not have concerns or they do not pay attention.
Methods:
In the two cases presented here, the patients did not have any pain, nor did they notice when the lesions appeared. These patients used conventional cigarettes for some time and then switched to smokeless tobacco due to relocation to Latvia. Soft tissue excision was performed and sent for histopathological examination.
Results:
The findings were proliferation of oral epithelial cells from buccal region, their overgrowth, an excessive amount of fibroblasts, cell destruction and necrosis, and a large amount of inflammatory cells, eosinophil leukocytes, and plasma cells.
Conclusion:
We can conclude that these intraoral findings are important risk factors for possibly developing precancerous lesions. Such mucosal changes can occur with different forms of tobacco, including Swedish snus and betel leaves. Dental practitioners should always question patients about tobacco use and regularly check for mucosal changes for early detection.
1. INTRODUCTION
Every year tobacco kills seven million people directly and 1.2 million people due to secondhand smoke [1]. New tobacco products such as heating devices, electronic delivery systems, and smokeless tobacco products are offered as a “healthier” alternative to conventional tobacco products such as cigarettes [2].
Smoke-free policies have been introduced to reduce the secondhand smoke risk for people in public areas [3]. This has also changed the behaviour of tobacco users, as they tend to use smokeless products, such as snus, nicotine pouches, or odourless products such as electronic cigarettes.
Based on the National Youth Tobacco Survey from the USA in 2021, e-cigarettes were the most commonly used product for middle school and high school students. In total, 13.4% of high school students and 4% of middle school students admitted that they used tobacco products daily; however, overall, tobacco products had been used by 34% of high school students and 11.3% of middle school students [4]. Among adolescents aged 13-15, the worldwide prevalence of using tobacco products other than cigarettes is increasing [5]. Data from the 2019 National Health Interview Survey showed that cigarettes are still the most popular tobacco product used (14%) in the USA among adolescents. E-cigarettes were used by 4.5% of respondents and smokeless tobacco by 2.4%. The average age for e-cigarette users was 18-24 years old, and half of them had not tried conventional cigarettes before, which means that tobacco trends are changing, and more people are attracted to novel tobacco products [6].
Secondhand and thirdhand smoke is significant not only when conventional cigarettes are used but also when e-cigarettes are used [7]. Thirdhand smoke is not only inhaled but also can be dermally absorbed through hand-to-mouth or object-to-mouth contact [8].
Smokeless tobacco (snus) products are products that are not involved in the combustion process [9]. Sales of tobacco for oral use are prohibited in the European Union except for Sweden, Finland, and Austria [10]. Although snus sales are prohibited, there are many young people that use snus products illegally [11].
Snus is a cut, moist tobacco product that can be in loose form or portioned in sachets that are placed under the upper or lower lip [12]. There are different types of snus: Swedish snus, American snus, Naswar, Toombak, Gutka, etc. Each type has different contents, pH levels, nicotine amounts, etc. Naswar, Toombak, and Gutka are frequently used in Sudan, India, and Pakistan, but Swedish snus is mainly used in Europe [13]. Swedish snus has higher levels of unionised nicotine compared to American snus. It is concluded that Swedish snus is more addictive and usually used as the main product, but American snus is lighter, and some users use it combined with cigarettes [14].
Nicotine pouches are white portioned pouches that contain nicotine, aromas, water, acid regulators, sweeteners, etc., but do not contain tobacco. They are sold in small containers and available in different flavours and strengths. The nicotine level per pouch ranges from 4mg/g to 34mg/g [15]. Manufacturers advise putting one pouch under the upper lip for 30 min. During this time, nicotine is absorbed through the mucosa, and the user feels tingling in the gums. Every container also has a side compartment where used pouches can be placed. All the containers are usually bright colours. The average package costs approximately EUR 5 in Latvia [16].
There are many products that can be used for vaping, and they come in different kinds of forms and sizes. There can be e-cigarettes, vapes, vape pens, dab pens, tanks, mods, pod mods, and electronic nicotine delivery systems. First generation e-cigarettes are disposable e-cigarettes that are designed to look like combustible cigarettes. They are not rechargeable or refillable. Second-generation e-cigarettes are rechargeable and designed to be used many times. The e-liquid comes in prefilled or refillable cartridges that are attached to a battery. Third-generation e-cigarettes are tanks or modifiable devices that allow users to customise the substances in the device. They are designed to be used multiple times. Fourth-generation e-cigarettes are pod mods, prefilled or refillable pod cartridges with a modifiable system. They are available in different shapes, colours, and sizes [17].
Young people use disposable pod e-cigarettes more often because of their relatively low price, and no other devices or liquids need to be purchased. For example, one disposable pod e-cigarette (Salt Switch) costs approximately EUR 6 per pod. Each pod has 2 ml of nicotine, and the manufacturers advertise that it will be enough for 450 puffs. Different kinds of flavours are available [18].
Tobacco heating devices are devices that heat but do not burn the tobacco leaves. The US FDA categorises tobacco heating devices under cigarettes [19]. One of the most popular heating device manufacturers advertises that heating devices are healthier than regular cigarettes [20].
For dental practitioners, it is difficult to question patients about tobacco consumption because, firstly, patients tend to not say what kind of products they are using, because some of them are illegal; secondly, sometimes patients switch tobacco products, for example, during weekdays, they use smokeless tobacco, but at weekends, they smoke or use more than one tobacco product [21].
Patients usually do not have any complaints about oral mucosal changes, because there is no pain in the early stages of the patient uses smokeless tobacco, which is why dental practitioners should be very careful to search for oral mucosal changes, first, visually, and second, by taking a biopsy for histopathological examination. Early clinical signs are non-healing ulcers and red or white patches in the mouth [22, 23].
Smokeless tobacco users tend to have white lesions such as parakeratosis, leucoplakia, leukoedema, smokeless tobacco keratosis, etc., at the places where the smokeless tobacco sachets are placed [24]. Tobacco use is a risk factor for oral squamous cell carcinoma [25]. Every year more than 350 000 oral cancer cases are diagnosed worldwide. The highest prevalence is in Asia, Europe, and North America [26]. Tobacco use is a risk factor for oral cancer [27].
The two cases described here are unique cases where dental practitioners detected oral mucosal changes in smokeless tobacco users visually and with histopathological examination. There is a rising interest in these novel products, which is why we should pay attention to their influence on oral health. As the products are quite new in the market, not much research has been conducted on this topic.
These two case reports were approved by the Ethics Committee of Rīga Stradiņš University No. 22/28.01.2016, and both participants signed informed consent for collecting biological material, publishing photographs of their oral cavity and giving their consent for publication of this study. The guidelines of the Helsinki Declarations complied. CARE guidelines were followed for the case series.
This research has been developed with financing from the European Social Fund and the Latvian state budget within project no. 8.2.2.0/20/I/004 “Support for involving doctoral students in scientific research and studies” at Rīga Stradiņš University.
2. PATIENT INFORMATION
Patient Nr.1 was a 67-year-old man. The patient did not take any medications or have systemic diseases. He smoked conventional cigarettes for most of his life (10 cigarettes per day); then, he started to use Swedish snus because of relocation to Scandinavia. For Swedish snus, he regularly used five sachets daily for at least five years. The patient did not have any complaints about his general health. The main dental complaint was increased mucosal tissue on the left cheek inside the oral cavity. The patient could not remember when the tissue enlargement appeared. He did not have any discomfort or pain. The patient denied any trauma that would create mucosal changes.
Patient Nr.2 was a 65-year-old man. The patient did not take any medications or have systemic diseases. The patient chewed betel leaves for 25 years; then, he switched to Swedish snus because of their easier availability in Europe. The main dental complaint was discomfort and a burning sensation in the tongue, but he did not have any pain. The patient denied any trauma that would create mucosal changes.
3. CLINICAL FINDINGS
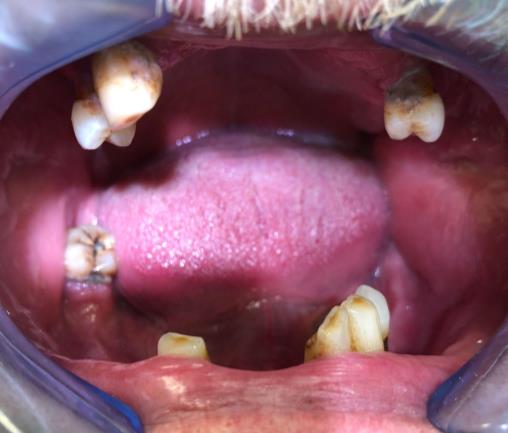
Patient Nr.1 had an elevated yellowish ulcer on the left buccal mucosa, 0.7 cm in diameter. It was not painful when palpating. He had partial adentia, with teeth that had calculus, plaque, and caries. The patient did not wear or have dentures (Fig. 1).
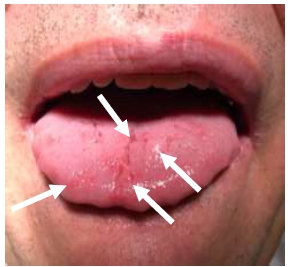
Patient Nr.2 had a fissured tongue, with two enlarged mobile papillae and red lesions on the tip of the tongue. The patient had full dentition, no caries, and good oral health (Fig. 2).
4. DIAGNOSTIC ASSESSMENT
For both cases, infiltration anaesthesia was made (Septanest forte 40mg/10μg/ml 1.0 ml), with soft tissue excision (5 mm in diameter, 3 mm thick) with a sterile scalpel. The tissue was placed in an Eppendorf Tube (5.0ml, Sigmaaldrich) with formaldehyde solution with a ratio of 1:15; then, it was stained with haematoxylin and eosin and examined with a microscope with a magnification of 40x, 100x, and 600x. The patients were instructed about preventive oral care after the biopsy.
5. HISTOPATHOLOGICAL FINDINGS
5.1. Patient Nr 1
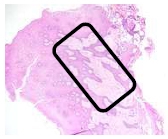
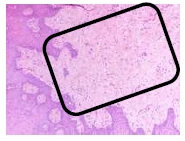
The findings were cell destruction and apoptosis, excessive proliferation of oral epithelial tissue, and four times thicker epithelial tissue compared to normal buccal tissues (Fig. 3). An excessive amount of fibroblast was seen (Fig. 4).
5.2. Patient Nr 2
There were no findings of oral candidosis. There was cell destruction and necrosis, with a large amount of inflammatory cells and eosinophil leukocytes (Figs. 5-7).
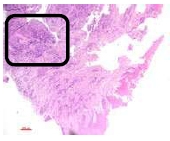
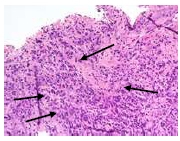
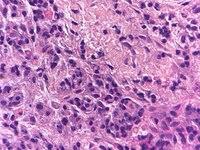
6. DISCUSSION
As seen from the histopathological findings, the smokeless tobacco user had excessive oral epithelial proliferation four times thicker than it should be. It has been concluded that nicotine stimulates normal cell and cancer cell proliferation [28, 29]. Buccal cells, when exposed to carcinogens show early signs of the carcinogenesis process, such as increased cell proliferation, decreased expression of E-cadherin, and alterations in the maturation pattern [30].
Our findings show that there was an inflammatory process where the tobacco was placed. Compared to other studies, we can say that smokeless tobacco changed the oral cells histologically with inflammation, binucleation, atypia, and keratinization [31].
Snus use has been associated with squamous cell carcinoma, verrucous carcinoma, tobacco pouch keratosis, leukoplakia, erythroplakia, and oral submucous fibrosis. Snus usage stains teeth discolours dentures and other prosthetic work, and increases the risk of caries, gingivitis, periodontitis, and gingival recessions [32]. Snus-related oral cavity cancer was higher among women than men [33]. Adults who used smokeless tobacco had a higher frequency of micronuclei. Micronuclei are considered an early marker of cancer [34]. Khan et al. concluded that there was a higher development of oral submucous fibrosis, an extremely high risk for oral cancer and a moderate risk for oral leucoplakia [35].
Smokeless tobacco contains several carcinogens but also tobacco-specific nitrosamines (TSNa), and their levels vary according to the type of smokeless tobacco [36]. Tobacco-specific nitrosamines could be used as a biomarker in oral fluids to detect cancer risk [37, 38].
This case series had several limitations; for example, the patients did not use just one product for the entire period. Although we cannot conclude that the mucosal changes were entirely from Swedish snus use, we can say that tobacco use (Swedish snus and other smokeless tobacco products) is a risk factor for developing mucosal changes. It should also be taken into account that there are many smokeless tobacco products that are popular in different regions of the world; for example, Swedish snus is more popular in Europe, but naswar or betel leaves are more popular in Asia, and content, manufacturing, and characteristics of each are different [39]. Moreover, oral cancer risk varies according to the smokeless tobacco used. The smokeless tobacco used in Asia poses a greater risk for developing oral cancer [13,40]. There is also a relevant risk that patients may have concealed that they have other medical problems or systemic diseases that would be a risk factor for mucosal changes.
CONCLUSION
We can conclude that these intraoral findings are an important risk factor for possibly developing precancerous lesions. Such mucosal changes can happen using different forms of tobacco, including Swedish snus and betel leaves. Novel tobacco forms are becoming more prevalent, and people are changing from conventional cigarettes to novel tobacco products. There is not as much research on the effects of novel tobacco products compared to conventional cigarettes. Dental practitioners should always question patients about tobacco use and regularly check for mucosal changes to aid early detection.
ETHICS APPROVAL AND CONSENT TO PARTI-CIPATE
Case reports were approved by the Ethics Committee of Rīga Stradiņš University No. 22/28.01.2016,.
HUMAN AND ANIMAL RIGHTS
No animals were used for studies that are the basis of this research. All the humans were used in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013 (http://ethics.iit.edu/ecodes/node/3931).
CONSENT FOR PUBLICATION
Both participants signed an informed consent for collecting biological material, publishing photographs of their oral cavity, and gave their consent for publication of this study.
STANDARDS OF REPORTING
CARE guideline has been followed.
FUNDING
This research has been developed with financing from the European Social Fund and the Latvian state budget within project no. 8.2.2.0/20/I/004 “Support for involving doctoral students in scientific research and studies” at Rīga Stradiņš University.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We are grateful for help and support from the Department of Biology and Microbiology, Rīgas Stradiņa University.


