All published articles of this journal are available on ScienceDirect.
Comparison of Sub-epithelial Connective Tissue Graft and Platelet Rich Fibrin in Peri-implant Soft Tissue Augmentation: A Randomized Clinical Split-mouth Study
Abstract
Aims and Background:
Gingival phenotype has a crucial impact on the peri-implant marginal bone stability. The aim of this clinical trial is to assess and compare the efficacy of the sub-epithelial connective tissue graft (SCTG) and platelet-rich fibrin (PRF) in improving the peri-implant soft tissue phenotype and enhancing esthetic outcomes.
Materials and Methods:
The present study was a split-mouth randomized controlled clinical trial. A total of ten patients who had bilateral missing teeth in the maxillary esthetic zone with a thin gingival phenotype were included in this study. For each study participant, one randomly selected site was treated with SCTG, while the other was treated with PRF membrane during dental implant placement. Treatment outcomes included the assessment of the facial gingival thickness using cone-beam computed tomography (CBCT) at the baseline (T0) and 6 months postoperatively (T1), and the Pink esthetic score (PES) at T1 and 3 months later after prosthesis placement (T2).
Results and Discussion:
Both treatment options resulted in a significant increase in gingival tissue thickness at T1 compared with T0, and in PES at T2 compared with T1 (p ˂ 0.05).
Conclusion:
PRF is an effective alternative to SCTG in augmenting peri-implant soft tissue phenotype and improving esthetic outcomes. This would help overcome the complications associated with harvesting the SCTG and increasing patients’ satisfaction.
Clinical Trial Registration ID: ISRCTN11961919.
1. INTRODUCTION
Dental implants are widely accepted as a treatment modality to replace missing dentition [1]. The increased patients’ esthetic expectations represent a critical parameter for implant success, especially in the esthetic zone [2]. Therefore,
the presence of healthy tissues at the implant soft tissue interface is recommended to support the long-term success and stability in function and esthetics [3].
In some situations, the esthetic outcomes of the implant therapy may be compromised due to lack of facial soft tissue dimensions (i.e. width and thickness) around the dental implant [4]. This is usually more pronounced in patients who do not maintain adequate oral care and show high levels of dental plaque accumulation [5], which is the major factor for initiating peri-implant inflammation [6]. In addition, there is an exaggerated risk of the gingival recession that results in metal exposure and renders implants esthetically unacceptable to the patients [3].
Initial mucosal thickness has a crucial impact on the peri-implant marginal bone stability [7]. It has been illustrated that thick gingival phenotype (i.e. ≥ 2 mm) is associated with reduced marginal bone loss in the first year after delivery of prosthesis and superior esthetic outcomes, due to less soft tissue discoloration, compared with thin mucosa [8, 9]. For this purpose, gingival augmentation is recommended to improve the peri-implant soft tissue thickness, which can be accomplished either prior to implant placement, simultaneously at the time of implant placement, or during the healing phase [10]. In this regard, various successful grafting materials have been proposed for soft tissue management. Thus, providing greater flexibility for the choice of the reconstruction material to obtain better aesthetic outcomes with respect to the color of peri-implant tissues [11].
Subepithelial connective tissue graft (SCTG) is the best choice for peri-implant soft tissue augmentation [12]. From the biological point of view, SCTG has the potential to induce the differentiation of mesenchymal cells into fibroblasts, which promotes epithelial proliferation and, consequently, helps modulate the soft tissue phenotype [13]. Nevertheless, post-operative donor-site morbidity, limited availability of the graft tissue, and the possible patient’s discomfort at the second surgical site are the main drawbacks of such treatment modality [14].
Platelet rich fibrin (PRF) has been introduced as an alternative to the SCTG to augment the gingival phenotype. It consists of a fibrin network containing platelets and a variety of growth factors, including transforming growth factor-beta1 (TGF-β1), platelet-derived growth factor (PDGF), Vascular endothelial growth factor (VEGF) [15]. These molecules are slowly released and act directly to promote the proliferation and differentiation of fibroblasts [16]. Even though the efficacy of applying PRF membrane in improving peri-implant soft tissue phenotype has been reported [17], more research work is still required to investigate its clinical performance.
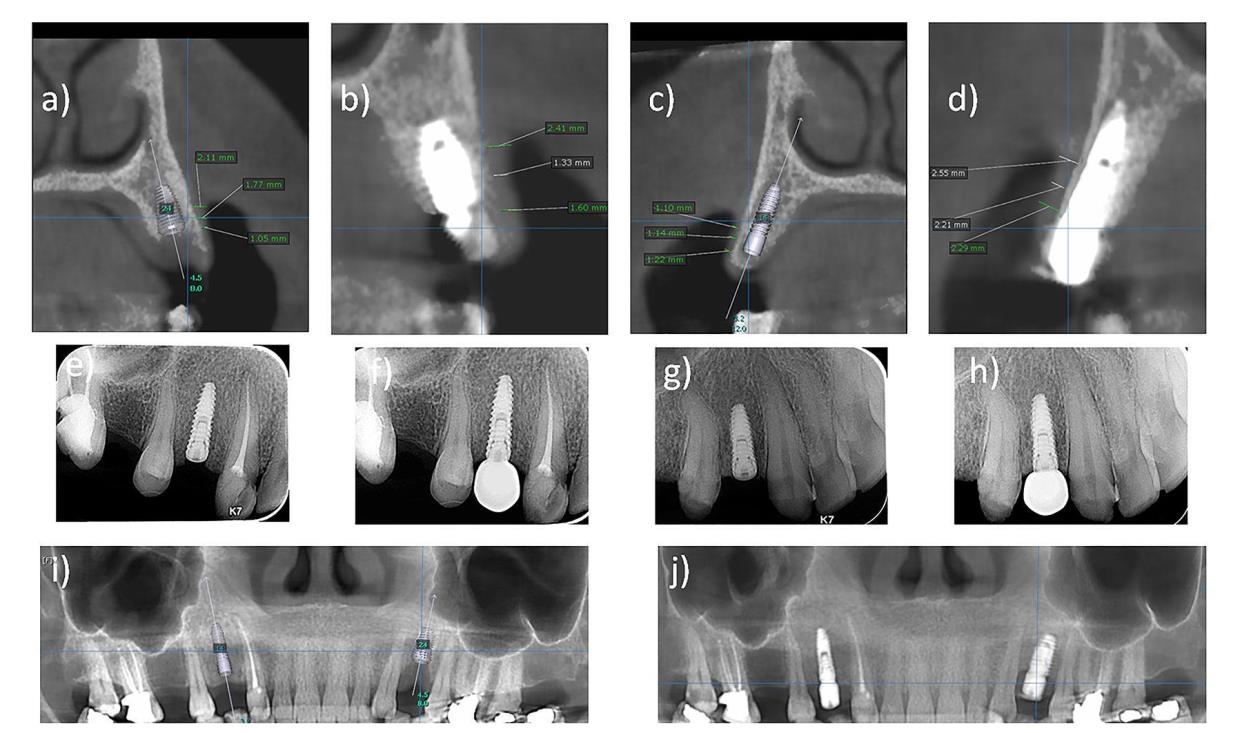
Different methods have been introduced to assess gingival thickness [18]. The direct (i.e. transgingival) method is the most commonly used [19, 20]; however, it presents limitations such as the possible low precision of periodontal probes and provoking discomfort for patients [21]. Although the application of the ultrasound approach seems to be effective in measuring the soft tissue thickness [22, 23], it does not permit the reproducibility of the calibration [23]. Recently, the use of cone-beam computed tomography (CBCT) has been suggested [24, 25], but the difficulty to establish limits between soft tissues and the vestibular bone crest may interfere with its accuracy in determining the gingival thickness. To overcome this limitation, it has been recommended to use a labial retractor during the exam to facilitate the visualization and measurement of soft structures of the periodontium [24]. The efficacy and reliability of applying CBCT in the assessment of gingival thickness have been well reported [26, 27].
In the current study, we hypothesized that PRF could be an effective alternative to SCTG in the augmentation of the peri-implant soft tissue thickness. Therefore, the aim of the present work was to compare the use of SCTG versus PRF, using a split mouth design, in terms of the peri-implant soft tissue thickness and the esthetic outcome of soft tissue around implant-supported single crowns.
2. MATERIALS AND METHODS
In this study, we aimed to test the impact of using either SCTG or PRF on improving the peri-implant soft tissue phenotype and the esthetic outcomes of implants placed in the esthetic zone. For this purpose, we conducted a split mouth design in which the implant site on one side was treated with PRF and that on the contralateral side was treated with SCTG. Evaluation of both soft tissue thickness and pink esthetic score was performed to compare the efficacy of the applied grafting methods. Below we provide specific details on the steps followed in our study.
2.1. Study Subjects
The current clinical study was approved by the Review Board in the Faculty of Dentistry, Mansoura University and was registered as a clinical trial with the ID (ISRCTN11961919). Participants in this study were selected from patients seeking dental implant replacement therapy and reporting to the dental clinic in the Periodontics Department, Faculty of Dentistry in Mansoura University, in the period between 2017-2020. The methodology was reviewed by an independent statistician.
2.2. Selection Criteria
Patients were included in the current study based on the following criteria:
2.2.1. Inclusion Criteria
1. Bilateral missing teeth in the maxillary anterior and premolar area.
2. Facial thin gingival phenotype facially (i.e. ˂ 1.5 mm) as evaluated using cone beam computed tomography (CBCT) [28].
3. Bilateral edentulous sites dimension of at least 5.5 mm bucco-lingually, 5.5 mm mesio-distally, and with a minimal bone height of 8 mm.
4. Teeth adjacent to the selected edentulous site must be free of periodontal disease involvement.
5. Adjacent teeth permit occlusal guidance.
6. An opposing dentition to the edentulous area with teeth, implants or fixed prosthesis.
2.2.2. Exclusion Criteria
1. Untreated rampant caries and/or uncontrolled periodontal disease.
2. Insufficient inter-occlusal distance for implant placement and restoration.
3. Smokers.
4. Systemic diseases are contraindicating dental implant placement like osteoporosis and uncontrolled diabetes mellitus.
5. History of radiation in the head and neck region.
6. Pregnancy.
7. Uncooperative patient.
2.3. Surgical Procedures
2.3.1. Preoperative Measures
Diagnostic impressions and study casts mounted on simple hinge articulator were used as a pre-treatment record to evaluate the possible prosthetic options in terms of occlusion, crown height space, and teeth inclination. Preoperative intraoral photographs were taken with a digital camera (D5200, Nikkor, Medical Objective ring flash; Nikon Corporation, Tokyo, Japan).
A written informed consent was signed by all patients and they were familiar with the possible post-surgical complications which may occur such as pain, post-operative bruising, and extra-oral swelling. Preoperative medications were prescribed, including prophylactic antibiotics (i.e., 2 gm amoxicillin, 1 h prior to the surgery).
2.3.2. Surgical Phase
2.3.2.1. Implant Placement
Based on the selection criteria and sample size calculation, a total of 10 patients were included in the current study. For each patient, two dental implants were planned to be placed, one on each side. The selected surgical sites were randomly assigned by using coin toss method by an independent person to be grafted with either SCTG or PRF.
The surgical procedures started with buccal and palatal infiltration anaesthesia using 4% articaine with 1:100.000 epinephrine. Crestal incision was made along the alveolar crest slightly toward the palate through keratinized attached mucosa. The incision was extended mesiodistally to the neighboring teeth for better visualization of the alveolar bone. The osteotomy was prepared according to the manufacturer's instruction to accommodate the selected implant size.
The acid etched, tapered, regular neck and bone level titanium dental implant (Neo Biotech IS II Active dental implant) was inserted with a minimum torque of 35–40 N-cm, and the implant was submerged in the osteotomy site with the average length of 10 millimeters and average diameter of 3.5 mm and a covering screw was placed (Figs. 1 and 2).
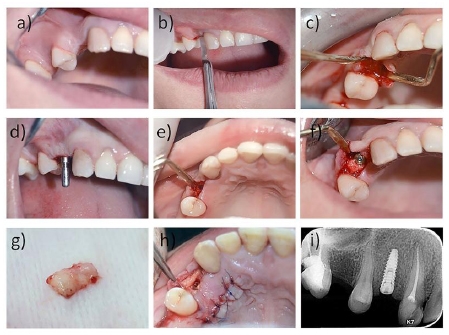
2.3.2.2. Grafting Materials
SCTG : the connective tissue graft was harvested following the parallel incision method which was developed by Langer et al. [29] (Fig. 1).
PRF : It was prepared by following the protocol developed by Choukroun et al. [30]. In brief, 10 cm blood specimen was collected from the patient in 10 ml dry plain glass test tubes (Marpe, Cairo, Egypt) without anticoagulants. The blood obtained from the candidate was placed immediately into the test tube and centrifuged at 3000 rpm for 10 minutes in a centrifuge machine (Spinplus Centrifuge:TC-SPINPLUS-6 Digital Desktop Centrifuge. TopScien, Zhejiang, China). This must be achieved immediately to prevent blood coagulation because of the absence of anticoagulant in the used test tubes. After that, the blood sample was separated into three layers; a layer of straw-colored acellular plasma at the upper fraction, fibrin clot at the middle fraction, and a layer of RBCs at the lower fraction.
Then, the upper portion of the test tube containing the acellular plasma is discarded. Also, the middle portion of the glass-test tube containing the fibrin clot is removed and scrapped off from the lower part containing the red blood cells which aren't of significant importance in the preparation of PRF. After that the PRF obtained was squeezed between two pieces of glass slides to obtain the PRF membrane (Fig. 2). Only one PRF membrane of a definite thickness, almost similar to that of the obtained SCTG, was then applied on the facial surface of the implant site.
2.3.2.3. Recipient Site Preparation for Graft Placement
A subperiosteal pouch or tunnel was made to allow application of the PRF or the SCTG on the facial aspect of the dental implant. A 5-0 Glycolon suture material was used to fix the grafting material to the overlying mucoperiosteum to ensure their stability during the healing period. To ensure intimate closure of the surgical site, simple interrupted sutures were also applied using a 5-0 Polypropylene suture material (Figs. 1 and 2).
2.3.3. Postoperative Follow-up
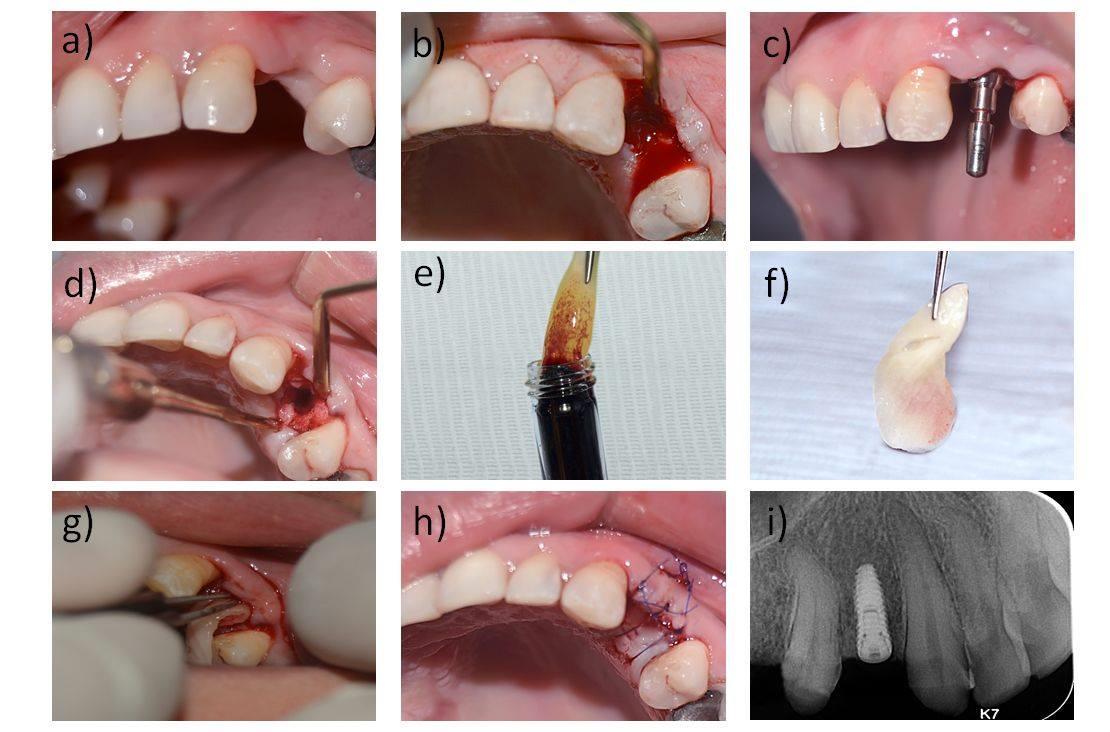
Post-operative medications, including antibiotic (1gm amoxicillin BID for 5 days), and analgesic (50 mg Diclofenac potassium TID for 3 days), were prescribed. Patients were instructed to maintain oral hygiene with Chlorhexidine digluconate mouth rinse (0.12%) the day after surgery. Sutures were removed 10 days after surgery. All patients were seen once monthly for six months following the surgical procedures to enhance oral hygiene measures and for regular assessment of the surgical sites. Six months after the surgical procedures, the patients were recalled to proceed with the prosthetic phase of treatment and receive their porcelain fused to metal prosthesis (Fig. 3).
2.3.4. Parameters Assessment
This was performed by an independent examiner, who was blinded to the method of soft tissue grafting used on each side. The peri-implant soft tissue thickness was evaluated at the baseline (T0) and 6 months postoperatively (T1) using CBCT as previously described [24]. The pink esthetic score (PES) integrates seven variables for a simple and clinically practiced evaluation with a 2–1–0 score rating system [31]. The assessment of this score was performed at 6 months postoperatively (T1) and 3 months later (T2) (Fig. 4).
3. STATISTICAL ANALYSIS
This was carried out by considering a power of 90% and a significance level of 0.05 alpha error to reject the null hypothesis that there were no differences between the grafting material used regarding their impact on esthetic outcomes and gingival augmentation. A difference of 10% was considered clinically relevant based on a previous study [32]. A total number of 8 patients were considered to be adequate for performing the study. However, one patient was added to compensate for a 20% potential drop-out. Descriptive statistics were performed and the normal distribution of the data was tested using Shapiro-Wilk test. Data were analyzed for significant differences using paired t-test. Statistical significance was set at p < 0.05. Data were analyzed using the SPSS software version 23.0 (v. 23, IBM Corp.; New York; USA).
4. RESULTS AND DISCUSSION
A total of ten patients, in the period between the years 2017 to 2020, were included in this study with an age ranging from twenty to forty-five years (7 females (70%) and 3 males (30%)). It was found that there was no significant difference between the thickness of the facial gingival tissues in both sides of the study group patients at the base line (T0) with P ˃ 0.05 (Table 1).
| - |
SCTG
(n = 10) |
PRF (n = 10) |
P value |
|---|---|---|---|
| Age/years | 30.50 ± 5.82 | 30.50 ± 5.82 | N/A |
| Average thickness of facial gingiva at T0 | 0.96 ± 0.13 | 0.94 ± 0.08 | P ˃ 0.5 |
Regarding the peri-implant soft tissue phenotype, a significant increase in the facial tissue thickness was detected with both treatment options at T1 compared with T0. However, inter-modality comparison at T1 revealed a significantly higher enhancement of tissue thickness with SCTG (2.98 mm ± 0.23) than with PRF (1.88 mm ± 0.14) (P < 0.05) (Table 2).
| - |
SCTG
(n = 10) |
PRF (n = 10) |
P value |
|---|---|---|---|
| T0 | 0.96 ± 0.12 | 0.94 ± 0.08 | P ˃ 0.5 |
| T1 | 2.98 ± 0.23 | 1.88 ± 0.14 | P ˂ 0.5 |
| P value | P < 0.05 | P < 0.05 | - |
On the other hand, significant improvement in the PES was obtained with both grafting options at (Ts) and (T1) (P < 0.05). However, the score measurements were found to be significantly better at sites grafted with SCTG (12.20 ± 0.63) than those treated with PRF (9.90 ± 1.1) (P < 0.05) (Table 3).
| - |
SCTG
(n = 10) |
PRF
(n = 10) |
P value |
|---|---|---|---|
| T1 | 5.1 ± 0.99 | 3.5 ± 1.1 | P ˂ 0.5 |
| T2 | 12.20 ± 0.63 | 9.90 ± 1.1 | P ˂ 0.5 |
| P value | P < 0.05 | P < 0.05 | - |
5. DISCUSSION
An implant therapy is considered successful when it fulfills not only the functional requirements but also the aesthetic outcomes which necessitate the presence of healthy and stable peri-implant tissues. A thin gingival phenotype is a crucial component correlated with facial soft-tissue recession. To mitigate the risk of developing undesirable changes of the soft-tissue margin, peri-implant soft tissue augmentation is usually suggested as a prophylactic measure. The present study demonstrated that both investigated grafting options; SCTG and PRF, were able to promote the peri-implant soft tissue phenotype and ameliorate the aesthetic outcomes.
Previous studies illustrated that autogenous soft tissue grafting is more effective in increasing soft tissue thickness than soft tissue substitutes [ 33,34]. A recent systematic review showed superior improvement in the gingival thickness obtained with the addition of an SCTG to the coronally advanced flap than with PRF [35]. These findings are consistent with our results which demonstrated a significant increase in the facial gingival tissue thickness at the sites treated with SCTG compared with those treated with PRF at 6 months postoperatively.
On the other hand, SCTG has been demonstrated to provide a substantial increase in the buccal peri-implant soft tissue. In this context, A randomized clinical trial reported an augmentation of 1.2 mm in the keratinized tissues thickness 3 months postoperatively [36]. Similarly, an increase of 1.3mm in soft tissue thickness was observed 1 year after augmentation with SCTG simultaneously during implant placement [37]. The present work revealed an increase of 2 mm following the application of CTG. The variation in the outcomes is likely attributed to the method used for the assessment of mucosal thickness. Previously, clinical assessment was usually used through transmucosal probing performed at a single or several points. Whilst, the present study relied on the radiographic evaluation using CBCT.
According to our results, a significantly higher increase in the soft tissue thickness could be detected in SCTG group than in the PRF counterpart. This outcome might be attributed to the structural characteristics of the grafts. The influence of underlying connective tissue on epithelial cell differentiation has been well documented, suggesting that the placement of a SCTG stimulates the proliferation of the overlying epithelial cells [13]. In addition, the rapid integration and revascularization of the graft support the differentiation and growth of fibroblasts which secrete the organic matrix. These properties would eventually result in tissue volume augmentation [38].
PRF contains and liberates a group of growth factors which stimulate a cascade of reactions upon binding to the transmembrane receptors located on the external surface of the cell membranes [39]. This likely leads to the activation of an endogenous internal signal protein, which results in the expression of a normal gene sequence of cells, such as cellular proliferation, and matrix formation [30].
An acceptable esthetic outcome is critical in esthetically sensitive areas [40]. Therefore, a successful implant therapy must allow placement of restoration with adequately esthetic appearance [41]. In this context, the level of the peri-implant soft tissue is decisive for the ‘natural’ appearance of implant-supported single-tooth replacements. The PES is a reliable tool for evaluating the esthetic appearance of the soft tissue around single-tooth implant crowns [31].
In the present study, the PES score for mesial and distal papilla increased significantly at 3 months following the final restoration placement which is consistent with the findings reported by Lai et al. [ 42 ]. The reconstruction of periodontal attachment may contribute to this improvement. However, the relationship between periodontal attachment and the height of the papilla is still not clear.
CONCLUSION
According to the outcomes of our study, we conclude that both SCTG and PRF resulted in increasing the thickness of the gingival phenotype. However, SCTG showed better achievements in that parameter. Owing to the promising results obtained by PRF, it can be used as an effective alternative to the SCTG in the peri-implant soft tissue augmentation to improve of the final esthetic outcomes. In addition, decreasing the morbidity related to the second surgical site and thus, increasing the patient’s comfort and satisfaction.
Further studies on this topic are needed to clarify the reasons for these differences in correlation to the pathophysiology of PRF and SCTG. Furthermore, we suggest using different thicknesses or layers of PRF membranes as a trial to improve the final outcome.
LIST OF ABBREVIATIONS
| SCTG | = Sub-epithelial Connective Tissue Graft |
| PRF | = Platelet-rich Fibrin |
| CBCT | = Cone-beam Computed Tomography |
| TGF-β1 | = Transforming Growth Factor-beta1 |
| PDGF | = Platelet-derived Growth Factor |
| VEGF | = Vascular Endothelial Growth Factor |
| PES | = Pink Esthetic Score |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The current clinical study was approved by the Review Board in the Faculty of Dentistry, Mansoura University, Egypt (2019-112).
HUMAN AND ANIMAL RIGHTS
No animals were used for studies that are the basis of this research. All the humans were used in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013 (http://ethics.iit.edu/ecodes/node/3931).
CONSENT FOR PUBLICATION
A written informed consent was taken from all patients before enrollment in the study.
STANDARDS OF REPORTING
CONSORT guidelines were followed.
AVAILABILITY OF DATA AND MATERIAL
The data that support the findings of this study are available from the corresponding author, [I.M.A], on special request.
FUNDING
None.
CONFLICT OF INTERESTS
There is no conflict of interest to be reported by the authors regarding this article.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Dr. Hend Magdy Gomaa (Assistant professor, Public Health and Community Medicine Department, Faculty of Medicine, Mansoura University) for helping in reviewing the methodology and performing the statistical section of the current study. In addition, the authors are pleased to acknowledge 3D Scan Center, Mansoura, Egypt for technical support and financial sharing. The present work is part of the studies of the first author to obtain a doctoral degree at the Faculty of Dentistry, Mansoura University, Egypt.


