All published articles of this journal are available on ScienceDirect.
Evaluation of Water Sorption and Solubility of Nano Titania Enriched Glass Ionomer Cement Considering the Storage Solution and Time
Abstract
Background:
A biocompatible additive to glass ionomer cement types without affecting their stability in moisture and dry conditions in demand.TiO2NPs are stable and bioactive nanoparticles that improved the mechanical properties of GICs, but their impact on water sorption and solubility remains undetermined.
Purpose:
This study aimed to evaluate the water sorption/solubility of glass ionomer cement incorporating titanium dioxide nanoparticles (TiO2NPs) in different storage solutions over time.
Methods:
A total of 60 glass ionomer discs were fabricated, and they were divided into two groups (n=30); conventional glass ionomer (control), TiO2 NPs modified glass ionomer. Each group was subdivided into three subgroups according to the type of storage solution used (n=10); artificial saliva, mouthwash with alcohol, and mouthwash without alcohol. Water sorption% and solubility% were recorded after immersion of specimens in the storage solutions according to the subdivided groups; at 24hrs, 1 week, and 1 month.
Results:
TiO2NPs were associated with a significant decrease in sorption% in artificial saliva at 1 week, alcohol at 24hrs, and a significant decrease in solubility% in all storage solutions at 24hrs and artificial saliva at 1 week. There was a significant decrease in water uptake associated with both materials in artificial saliva, also with TiO2NPs modified type in alcohol-free mouth washes. There was a gradual significant increase in solubility% for conventional GIC in artificial saliva and TiO2NPs modified type in alcohol (p≤0.05).
Conclusion:
TiO2NPs may play a promising role in improving water sorption and solubility of conventional GIC, considering the type of storage solution and time.
1. INTRODUCTION
The resistance of the restorative material to intraoral conditions is very important for the longevity of the restorations. Materials that are placed for long periods in the oral environment undergo interactions with oral fluids. In some cases, the interaction may include dissolution or degradation of surface layers, whilst in others; the interaction may include the release of unbound or loosely bound components or uptake of fluids within the structure of the material [1].
The chemical stability in a wet environment is crucial to guarantee the viability of the materials by ensuring adequate mechanical properties, and a non-porous and smooth surface. Water sorption can increase the dimensions of the material, which can act as a plasticizer, leading to the deterioration of the matrix structure. The resulting dimensional changes lead to discolorations and breakage in the edge profile [1].
Solubility is a phenomenon defined as the degree to which a material dissolves in the solvent at a given temperature. It adversely affects the compatibility of restorations with biological structures and maximizes the rate of deterioration. Sorption and solubility of restorative materials depend on the features of solutions [2].
Glass ionomer cement types (GICs) are clinically attractive dental materials with unique properties that make them useful as luting materials, liners and bases, orthodontic bracket adhesives, core buildups, pit and fissures sealants, and restorative materials. This includes its coefficient of thermal expansion close to the tooth structure, biocompatibility, antimicrobial potential, adhesive strength, and anti-cariogenic capability [3].
Conversely, glass ionomer cements GICs suffer from clinical limitations such as low abrasion resistance, low fracture toughness, low mechanical properties, prolonged solidification rate, and high early moisture sensitivity [4].
Nano-dentistry is an emerging field in dentistry that uses nanostructured materials for diagnosing, treating, and preventing oral and dental diseases, relieving pain, and protecting dental health. Furthermore, nanostructured materials improved the properties of the materials. Efforts have been made to improve GICs’ physical and mechanical properties without affecting their biological properties by the addition of a variety of filler materials [5, 6].
TiO2 nanostructures are the subject of intense research due to their chemical stability and non-toxicity. It improved the mechanical properties of composites and hybrid materials [7, 8]. The majority of nanotechnology-based studies focused on assessing their effects on GICs’ mechanical performance, therefore the impact of TiO2 nanoparticles on GICs’ biocompatibility remains undetermined, as do their effects on GICs’ physical-chemical properties, their behavior in moisture and dry conditions [8, 9].
Nowadays, people use mouthwashes widely even without a dental prescription. The frequency of mouthwash use was up to six times/day [10]. Water, antimicrobial agents, salts, preservatives, and alcohol are the different components of a mouthwash. It has been reported that alcohol increases the degradation of restorative materials [11].
Most researchers studied the adsorption after immersion in water [12], artificial saliva [13], ethanol/water solution [14], and sodium chloride [15]. Since literature is spare in evaluating the effect of alcohol-containing mouthwash on the sorption of GICs, residence time in the storage environment may be crucial in assessing the clinical durability of restorative materials. In addition, there is no literature study to date comparing the effect of mouthwash on water sorption and solubility of TiO2NPs modified glass ionomer cement.
It is hypothesized that those TiO2NPs modified glass ionomer cement will show lesser sorption and solubility than the conventional type; therefore, the current study aimed to evaluate water sorption and solubility of TiO2NPs modified glass ionomer type in alcohol-containing mouthwash over time.
The tested null hypotheses of the current study were the following ones (1): There would be no statistically significant difference in water sorption and solubility values considering the tested materials, (2) there would be no difference between different storage solutions (Artificial saliva, mouthwash with alcohol (Hexitol), and mouthwash without alcohol (DG care), (3) Water sorption and solubility of glass ionomer material would not be affected by storage time.
2. MATERIALS AND METHODS
2.1. Materials
2.1.1. Kromoglass 2
Kromoglass 2 is water-based glass ionomer cement (mixable with water) for filling. It purchased from (LASCOD Spa-Via L.Longo, 18 50019 Sesto F.no (Firenze), Italy).
2.1.2. Titanium Dioxide Nanoparticles Preparation(TiO2NPs)
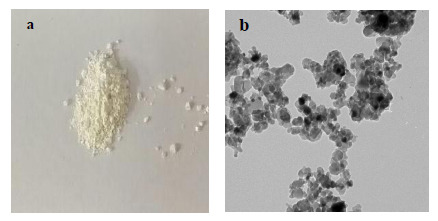
The anatase phase of TiO2NPs was successfully synthesized by the sol-gel method using titanium tetrachloride (TiCl4) as a precursor (Nano-Tech Company for Photo electronics, Egypt), followed by Dalvandi and Ghasemi, 2013 [16]. The resulting white powder was examined using a high-resolution transmission electron microscope TEM (JEOL, JEM-2100) at a voltage of 200KV. TEM micrographs showed that; the prepared TiO2NPs had a spherical shape as represented in Fig. (1). The size ranged from 25 ± 5 nm with purity >99.5%.
2.1.3. Storage Solution
Different storage media were used according to the subdivided groups. Storage solutions used in this study, type, and manufacturer are shown in Table 1.
2.2. Methods
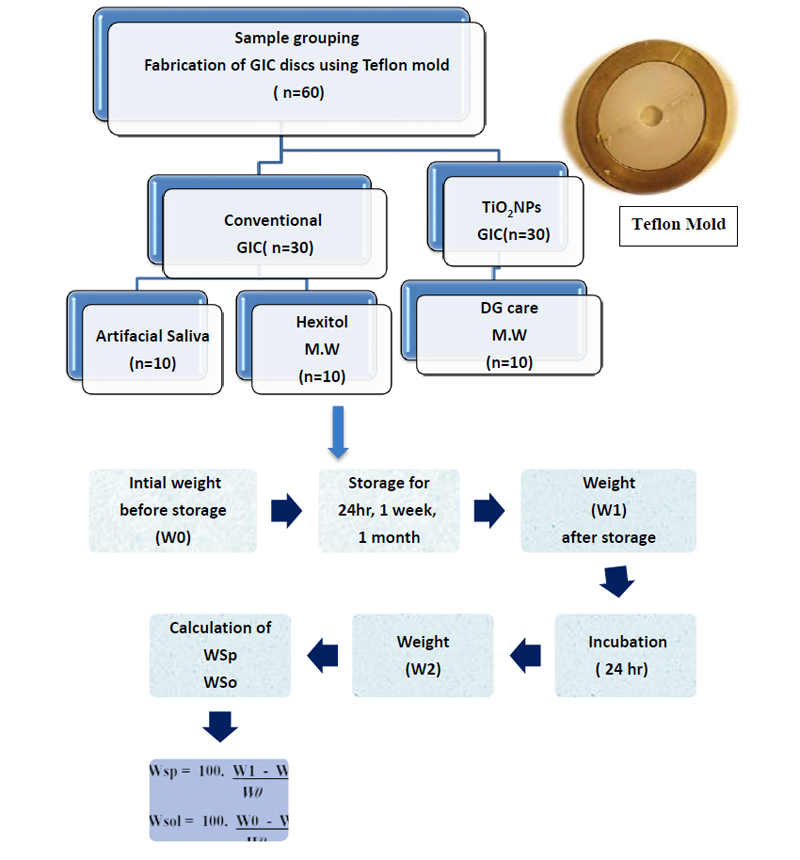
60 GIC discs were prepared from the tested materials. They were divided into two groups according to the incorporation of titanium dioxide nanoparticles (TiO2NP) into glass ionomer (n=30); conventional (control) and glass ionomer incorporating TiO2NP. Each group was subdivided into three subgroups according to the storage solution used (n=10); Artificial saliva, Hexitol (with ethanol 96%), and DG care (Alcohol-free) as represented in Fig. (2).
| Solution | Type | Composition | Manufacturer |
| Artificial saliva | Prepared, Storage medium | 9g NaCl+0.24g CaCl2+0.43g KCl + 0.2gNaHCO3 all dissolved in 1 L of water according to a previous study [17] | Prepared at AL-Azhar University Regional center for mycology and biotechnology Cairo, Egypt |
| Hexitol | Alcohol containing Mouthwash | 0.12% chlorhexidine as an active ingredient, glycerin, propylene glycol, Alcohol 96% (ethanol), Anise oil, Peppermint oil, Ponceau RH40, and pure water as inactive ingredients. | Arab Drug Company for Pharmaceutical and Chemical Industries, Cairo, ARE |
| DG care | Alcohol-free Mouthwash | Chlorhexidine gluconate 0.12% + Propolis 1% + Clove Oil 1%. | ALESRAA, Al Esraa Pharmaceutical Optima, Cosmetic Product, Egypt |
2.3. Incorporation of TiO2NPs into Conventional Glass Ionomer
Glass ionomer powder was blended with TiO2NPs powder at 10% (w/w). 0.5 g of TiO2NP powder was weighed using a digital balance sensor (GF-300, D448900640, AD Co., Ltd. Japan) with a precision of 0.0001g. The paper pad was applied on the digital balance then TiO2NPs powder was added gradually into the paper pad till the target weight was reached. 5g of GIC powder was weighed in the same manner and then blended with TiO2NPs using a vortex (RX3 Velp Scientifica, Milan, Italy) for one minute.
2.4. Preparation of Glass Ionomer Discs
GICs discs were prepared using a split Teflon mold with dimensions of 10 mm in diameter and a thickness of 2mm (Fig. 2). The powder and liquid were proportioned and mixed according to the manufacturer’s instructions. The mixed materials were inserted into the mold and placed on a transparent matrix and glass slide. The filled mold was covered with another transparent matrix strip and glass slide. Light pressure was used to extrude excess material from the mold to produce a smooth flat surface. Specimens were removed from the mold; each specimen was examined with the naked eye against the light to check for internal porosities.
2.5. Sorption and Solubility Test
Samples were initially weighted to an accuracy of 0.0001g using a digital (Analytical) balance. Each specimen was continuously weighed 3 successive times and the average weight was taken. The recorded mass was denoted as (W0). Immediately after weighing, specimens were placed in their storage environment according to the subdivision group. Each specimen was individually stored in a labeled, airtight glass container.
Each container contained approximately 10 ml of the test storage solution. Specimens were stored for 24 hours, 1 week, and 1 month at 37°C in an incubator, and the tested solutions were changed daily. By the end of each time interval, each sample was weighted. Before weighing, it was wiped lightly on filter paper until there was no visible surface moisture. It was then immediately weighed (W1) to eliminate the effect of dryness. All samples were then dehydrated in an incubator at 37°C for 24 hours and weighed again (W2) as represented in Fig. (2).
The amount of water sorption in each group was calculated from the difference between the initial mass and the wet mass for each sample after each storage period (W1 – W0). Material loss (solubility) was obtained from the difference between the initial dry mass and final dry mass values of each sample after each storage period (W0-W2). The percentage of water sorption (Wsp) and solubility (Wsol) for each sample were calculated using the following equations following a previous study [18].
Wsp = 100. W1 - W0/W0
Wsol = 100. W0 - W2/W0
2.6. Statistical Analysis
The statistical analysis software used was Statistical Package for Social Sciences (SPSS) version 18. Comparisons between groups for normally distributed numeric variables were compared by one-way analysis of variance (ANOVA) test, followed by Tukey’s post hoc test. A comparison of different observation times was performed by repeated measures ANOVA test. Comparison between material groups was performed by independent t-test.
3. RESULTS
3.1. Effect of incorporation of TiO2NPs on sorption and solubility rate
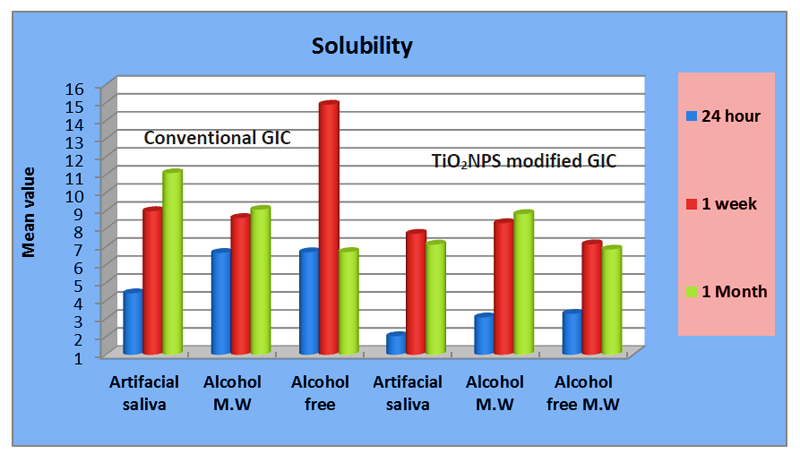
Descriptive statistics of water sorption (%-) and solubility (%) and a comparison between both types of glass ionomer using the (t-test) are summarized in Table 2. A graphical representation of water sorption% and solubility % is displayed in Figs. (3 and 4).
| - | Time | Material | Mean | Std. Dev | Mean Difference | t | P Value | |
|---|---|---|---|---|---|---|---|---|
| Artificial saliva | 24 hours | Sorption | Conventional Tio2 |
1.63 1.27 |
.88 .16 |
0.36 | .990 | .365 ns |
| Solubility | Conventional Tio2 |
4.41 2.03 |
1.10 1.84 |
2.39 | 2.726 | .025* | ||
| 1 week | Sorption | Conventional Tio2 |
1.27 -3.50 |
.18 .16 |
4.77 | 48.399 | .000* | |
| Solubility | Conventional Tio2 |
8.97 7.71 |
1.01 .13 |
1.27 | 3.064 | .027* | ||
| 1 month | Sorption | Conventional Tio2 |
-4.82 -6.35 |
2.13 .10 |
1.52 | 1.747 | .141ns | |
| Solubility | Conventional Tio2 |
11.08 7.11 |
1.06 .33 |
3.97 | 8.785 | .000* | ||
| Alcohol | 24 hours | Sorption | Conventional Tio2 |
2.59 1.91 |
.04 .50 |
0.68 | 3.355 | .020* |
| Solubility | Conventional Tio2 |
6.65 3.07 |
2.14 .72 |
3.58 | 3.876 | .008* | ||
| 1 week | Sorption | Conventional Tio2 |
-4.40 -4.97 |
1.59 .99 |
0.57 | .740 | .479ns | |
| Solubility | Conventional Tio2 |
8.59 8.30 |
.85 1.12 |
0.29 | .506 | .625ns | ||
| 1 month | Sorption | Conventional Tio2 |
-4.30 -5.68 |
.85 3.06 |
1.38 | 1.066 | .329ns | |
| Solubility | Conventional Tio2 |
9.05 8.81 |
2.88 .62 |
0.24 | .202 | .847ns | ||
| Alcohol- free | 24 hours | Sorption | Conventional Tio2 |
2.21 1.60 |
.63 .27 |
0.62 | 2.201 | .065 ns |
| Solubility | Conventional Tio2 |
6.69 3.27 |
1.35 .68 |
3.42 | 5.526 | .001* | ||
| 1 week | Sorption | Conventional Tio2 |
-3.34 -5.61 |
2.57 .34 |
2.27 | 2.142 | .083 ns | |
| Solubility | Conventional Tio2 |
14.88 7.13 |
8.95 .81 |
7.74 | 2.111 | .088 ns | ||
| 1 month | Sorption | Conventional Tio2 |
-5.66 -6.08 |
20.13 .70 |
0.43 | .052 | .961 ns | |
| Solubility | Conventional Tio2 |
6.70 6.84 |
17.61 1.03 |
-.14 | -.020 | .985 ns | ||
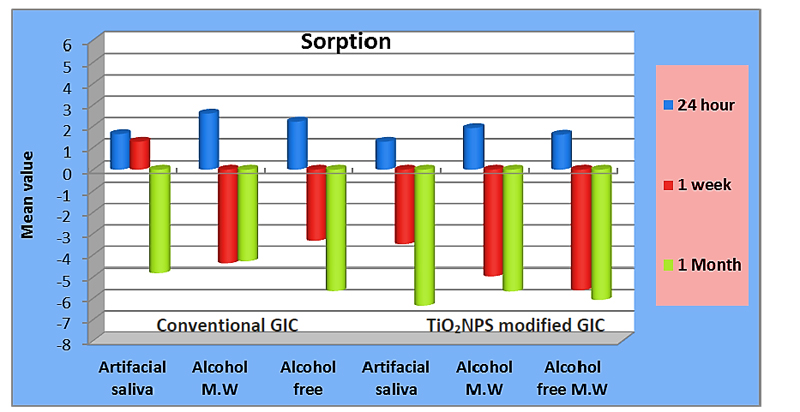
For water sorption, results showed higher statistically significant sorption (%-) with conventional glass ionomer in artificial saliva at 1 week and in alcohol at 24hrs (1.27 ± 0.18) and (2.59 ± 0.04), respectively, compared to the modified type which showed lower statistically significant sorption% in the same solutions (-3.50 ±0.16) and (1.91±0.50), respectively (p≤0.05).
On the other hand, the conventional type showed a higher non-significant sorption rate in both solutions at other time intervals. Similarly, it showed a higher none statistically significant sorption rate in alcohol-free mouthwash.
For water solubility (%), a higher statistically significant rate was associated with conventional GIC in artificial saliva at 24hrs., 1 week, and 1 month (4.41± 1.10), (8.97±1.01), and (11.8±1.06) respectively, and in alcohol and alcohol-free mouthwashes at 24hrs (6.56± 2.14), (6.69 ± 1.35) respectively, (p≤0.05).
On the other hand, results showed a non-statistically significant difference between the conventional type and the modified one in alcohol and alcohol-free mouthwashes at 1 week and 1 month.
3.2. Effect of storage solutions on water sorption and solubility
Descriptive statistics of water sorption (%), solubility (%), and comparison between storage solutions for conventional and TIO2NPs modified glass ionomer groups are summarized in Table 3a and 3b, respectively. A graphical representation of water sorption% and solubility % between different storage solutions is displayed in Figs. (3 and 4).
| Time | Mean | Std. Dev | 95% Confidence Interval for Mean | Min | Max | F | P value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||||||||
| 24 hours | Sorption | Artificial saliva Alcohol Alcohol-free |
1.63b 2.59a 2.21a,b |
.88 .04 .63 |
.71 2.55 1.55 |
2.56 2.63 2.87 |
.76 2.55 1.42 |
2.69 2.62 2.75 |
3.558 | .05* | |
| Solubility | Artificial saliva Alcohol Alcohol-free |
4.41y 6.65x 6.69x |
1.10 2.14 1.35 |
3.26 4.40 5.27 |
5.57 8.90 8.11 |
3.08 4.87 4.95 |
5.50 9.37 7.74 |
3.992 | .041* | ||
| 1 week | Sorption | Artificial saliva Alcohol Alcohol-free |
1.27a -4.40b -3.34b |
.18 1.59 2.57 |
1.08 -6.07 -6.04 |
1.46 -2.73 -.63 |
1.11 -6.43 -5.39 |
1.50 -3.12 -.05 |
17.765 | .000* | |
| Solubility | Artificial saliva Alcohol Alcohol-free |
8.97 8.59 14.88 |
1.01 .85 8.95 |
7.92 7.70 5.48 |
10.03 9.49 24.27 |
7.68 7.53 7.83 |
9.63 9.36 26.33 |
2.730 | .098 ns | ||
| 1 month | Sorption | Artificial saliva Alcohol Alcohol-free |
-4.82 -4.30 -5.66 |
2.13 .85 20.13 |
-7.06 -5.19 -26.78 |
-2.58 -3.40 15.47 |
-6.46 -4.95 -28.82 |
-2.08 -3.21 16.12 |
.021 | .980 ns | |
| Solubility | Artificial saliva Alcohol Alcohol-free |
11.08 9.05 6.70 |
1.06 2.88 17.61 |
9.97 6.03 -11.78 |
12.19 12.07 25.18 |
9.76 5.33 -14.20 |
12.04 11.04 24.90 |
.271 | .766 ns | ||
Tukey’s post hoc test: Within the same comparison, means sharing the same superscript letter are not significantly different
| Time | Mean | Std. Dev | 95% Confidence Interval for Mean | Min | Max | F | P value | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||||||
| 24 hours | Sorption | Artificial saliva Alcohol Alcohol- free |
1.27b 1.91a 1.60a,b |
.16 .50 .27 |
1.11 1.39 1.31 |
1.44 2.43 1.88 |
1.09 1.46 1.26 |
1.45 2.53 1.85 |
5.211 | .019* |
| Solubility | Artificial saliva Alcohol Alcohol- free |
2.03 3.07 3.27 |
1.84 .72 .68 |
.10 2.32 2.56 |
3.96 3.83 3.98 |
.67 2.43 2.41 |
4.39 3.97 3.81 |
1.846 | .192ns | |
| 1 week | Sorption | Artificial saliva Alcohol Alcohol- free |
-3.50a -4.97b -5.61b |
.16 .99 .34 |
-3.67 -6.01 -5.96 |
-3.33 -3.92 -5.25 |
-3.62 -5.98 -5.87 |
-3.30 -3.77 -5.17 |
18.530 | .000* |
| Solubility | Artificial saliva Alcohol Alcohol- free |
7.71 8.30 7.13 |
.13 1.12 .81 |
7.57 7.13 6.28 |
7.84 9.48 7.98 |
7.54 7.22 6.42 |
7.80 9.67 8.15 |
3.188 | .070ns | |
| 1 month | Sorption | Artificial saliva Alcohol Alcohol- free |
-6.35 -5.68 -6.08 |
.10 3.06 .70 |
-6.45 -8.90 -6.82 |
-6.24 -2.47 -5.35 |
-6.46 -8.52 -6.81 |
-6.24 -1.88 -5.25 |
.203 | .818ns |
| Solubility | Artificial saliva Alcohol Alcohol- free |
7.11y 8.81x 6.84y |
.33 .62 1.03 |
6.76 8.16 5.76 |
7.46 9.46 7.92 |
6.80 8.23 5.56 |
7.52 9.58 7.79 |
13.156 | .001* | |
Tukey’s post hoc test: Within the same comparison, means sharing the same superscript letter are not significantly different
For water sorption, results of the ANOVA test for both materials showed a statistically significant difference between all storage media at 24hrs (p≤0.05). The highest sorption rate was recorded with alcohol (2.59 ± 0.04), (1.91±0.50) for conventional and TiO2NPs modified types respectively. There was a statistically significant difference between all storage solutions at 1 week (p≤0.05), with artificial saliva, recorded the highest % (1.27 ± 0.18), (-3.50 ±0.16) for conventional and TiO2NPs modified types, respectively.
On the other hand, there was a non-statistical significant difference between all storage media at 1 month for both materials.
Post hoc test results showed no significant difference between alcohol-free and other storage solutions at 24hrs for both materials and 1 week for the conventional type. Furthermore, it showed no significant difference between alcohol-free and alcohol at 1 week for TiO2NPs type.
For water solubility (%), results of the ANOVA test for the conventional glass ionomer, showed a statistically significant difference between all storage media at 24hrs (p≤0.05), with alcohol-free recorded the highest solubility (%) (6.69 ±1.35). There was no statistically significant difference between all storage media at 1 week and 1 month.
On the other hand, for water solubility of TIO2NPs modified GIC, there was a statistically significant difference between all storage media at 1 month (p≤0.05), with alcohol recorded the highest rate (8.81 ± 0.62). There were no significant differences between storage solutions at 24hrs and 1 week.
Post hoc test results showed no significant difference between alcohol-free and alcohol for the conventional type at 24hrs, also between alcohol-free and artificial saliva at 1 month for the modified type.
3.3. Effect of Storage Time on Water Sorption and Solubility
Descriptive statistics of water sorption (%), solubility (%), and comparison between observation times for conventional and TiO2NPs modified glass ionomer groups are summarized in Tables 4a and 4b, respectively. A graphical representation of water sorption% and solubility % over time is displayed in Fig. (5a-d).
Results of the repeated measures ANOVA test showed a statistically significant decrease in water uptake by time for both materials stored in artificial saliva(p≤0.05), with the lowest sorption(%), recorded at 1 month (-4.82 ± 2.13, (-6.35 ± 0.1) for the conventional and modified types respectively. Similarly, a significant gradual decrease of sorption (%) associated with the modified type stored in alcohol and alcohol-free mouthwashes (p≤0.05), with the lowest sorption (%), recorded at 1 month (-5.68 ± 3.06), (-6.08 ± 0.70), respectively. On the other hand, the lowest sorption (%) for conventional GIC in alcohol was recorded at 1 week (- 4.40 ± 1.59).
| Storage Media | Mean | Std. Dev | Std. Error | 95% Confidence Interval for Mean | Min | Max | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | F | P | ||||||||
| Artificial saliva | Sorption | 24 hour | 1.63a | .88 | .36 | .71 | 2.56 | .76 | 2.69 | 44.20 | .000* |
| 1 week | 1.27a | .18 | .07 | 1.08 | 1.46 | 1.11 | 1.50 | ||||
| 1 month | -4.82b | 2.13 | .87 | -7.06 | -2.58 | -6.46 | -2.08 | ||||
| Solubility | 24 hour | 4.41z | 1.10 | .45 | 3.26 | 5.57 | 3.08 | 5.50 | 62.54 | .000* | |
| 1 week | 8.97y | 1.01 | .41 | 7.92 | 10.03 | 7.68 | 9.63 | ||||
| 1 month | 11.08x | 1.06 | .43 | 9.97 | 12.19 | 9.76 | 12.04 | ||||
| Alcohol | Sorption | 24 hour | 2.59a | .04 | .01 | 2.55 | 2.63 | 2.55 | 2.62 | 88.58 | .000* |
| 1 week | -4.40b | 1.59 | .65 | -6.07 | -2.73 | -6.43 | -3.12 | ||||
| 1 month | -4.30b | .85 | .35 | -5.19 | -3.40 | -4.95 | -3.21 | ||||
| Solubility | 24 hour | 6.65 | 2.14 | .87 | 4.40 | 8.90 | 4.87 | 9.37 | 2.15 | .151ns | |
| 1 week | 8.59 | .85 | .35 | 7.70 | 9.49 | 7.53 | 9.36 | ||||
| 1 month | 9.05 | 2.88 | 1.18 | 6.03 | 12.07 | 5.33 | 11.04 | ||||
| Alcohol- free | Sorption | 24 hour | 2.21 | .63 | .26 | 1.55 | 2.87 | 1.42 | 2.75 | .71 | .506ns |
| 1 week | -3.34 | 2.57 | 1.05 | -6.04 | -.63 | -5.39 | -.05 | ||||
| 1 month | -5.66 | 20.13 | 8.22 | -26.78 | 15.47 | -28.82 | 16.12 | ||||
| Solubility | 24 hour | 6.69 | 1.35 | .55 | 5.27 | 8.11 | 4.95 | 7.74 | 1.02 | .383ns | |
| 1 week | 14.88 | 8.95 | 3.65 | 5.48 | 24.27 | 7.83 | 26.33 | ||||
| 1 month | 6.70 | 17.61 | 7.19 | -11.78 | 25.18 | -14.20 | 24.90 | ||||
Tukey’s post hoc test: Within the same comparison, means sharing the same superscript letter are not significantly different
| Storage Media | Mean | Std. Dev | Std. Error | 95% Confidence Interval for Mean | Min | Max | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | F | P | ||||||||
| Artificial saliva | Sorption | 24 hour | 1.27a | .16 | .07 | 1.11 | 1.44 | 1.09 | 1.45 | 4383.5 | .000* |
| 1 week | -3.50b | .16 | .07 | -3.67 | -3.33 | -3.62 | -3.30 | ||||
| 1 month | -6.35c | .10 | .04 | -6.45 | -6.24 | -6.46 | -6.24 | ||||
| Solubility | 24 hours | 2.03y | 1.84 | .75 | .10 | 3.96 | .67 | 4.39 | 49.94 | .000* | |
| 1 week | 7.71x | .13 | .05 | 7.57 | 7.84 | 7.54 | 7.80 | ||||
| 1 month | 7.11x | .33 | .14 | 6.76 | 7.46 | 6.80 | 7.52 | ||||
| Alcohol | Sorption | 24 hours | 1.91a | .50 | .20 | 1.39 | 2.43 | 1.46 | 2.53 | 29.74 | .000* |
| 1 week | -4.97b | .99 | .41 | -6.01 | -3.92 | -5.98 | -3.77 | ||||
| 1 month | -5.68b | 3.06 | 1.25 | -8.90 | -2.47 | -8.52 | -1.88 | ||||
| Solubility | 24 hours | 3.07y | .72 | .29 | 2.32 | 3.83 | 2.43 | 3.97 | 84.21 | .000* | |
| 1 week | 8.30 x | 1.12 | .46 | 7.13 | 9.48 | 7.22 | 9.67 | ||||
| 1 month | 8.81x | .62 | .25 | 8.16 | 9.46 | 8.23 | 9.58 | ||||
| Alcohol- free | Sorption | 24 hours | 1.60a | .27 | .11 | 1.31 | 1.88 | 1.26 | 1.85 | 490.60 | .000* |
| 1 week | -5.61b | .34 | .14 | -5.96 | -5.25 | -5.87 | -5.17 | ||||
| 1 month | -6.08b | .70 | .29 | -6.82 | -5.35 | -6.81 | -5.25 | ||||
| Solubility | 24 hours | 3.27y | .68 | .28 | 2.56 | 3.98 | 2.41 | 3.81 | 38.23 | .000* | |
| 1 week | 7.13x | .81 | .33 | 6.28 | 7.98 | 6.42 | 8.15 | ||||
| 1 month | 6.84x | 1.03 | .42 | 5.76 | 7.92 | 5.56 | 7.79 | ||||
Tukey’s post hoc test: Within the same comparison, means sharing the same superscript letter are not significantly different.
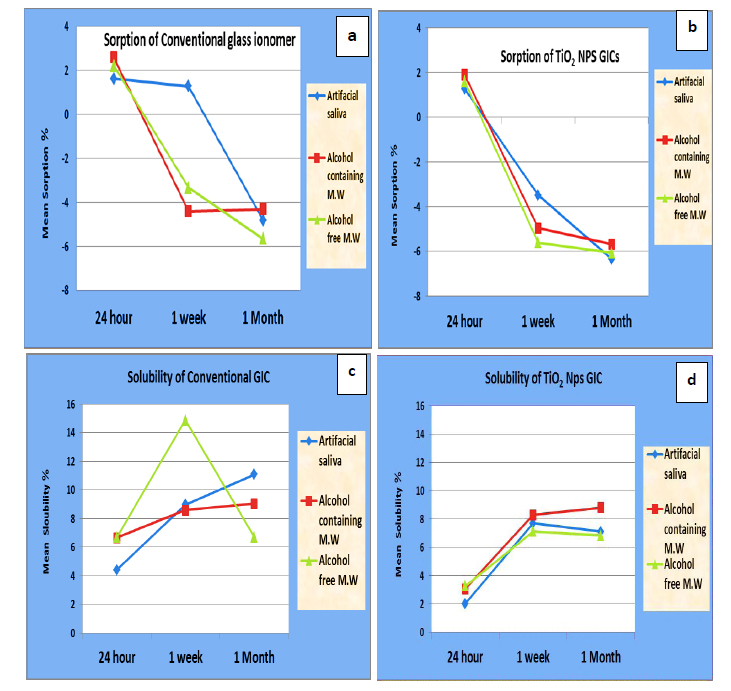
There was no statistically significant difference between all-time intervals with conventional type stored in alcohol-free mouthwash.
Post hoc test showed no significant difference between 24 hours and 1 week in artificial saliva for conventional type. It showed no significant difference between 1 week and 1 month for modified type in alcohol and alcohol-free mouthwashes.
Furthermore, results of the repeated measures ANOVA test showed a gradual statistically significant increase in solubility (%) by time with conventional GIC stored in artificial saliva(p≤0.05), with the highest solubility rate recorded at 1 month (11.8 ± 1.06). there was no significance difference between all-time intervals for conventional type in alcohol and alcohol-free.
There were statistically significant differences in solubility (%) between all-time intervals associated with TiO2NPs type in all storage solutions (p≤0.05), with the highest rate recorded at 1 month in alcohol (8.81 ± 0.62). On the other hand, the highest solubility (%) in artificial saliva and alcohol-free mouthwash was recorded at 1 week (7.71 ± 0.13) and (7.13 ± 0.81), respectively.
Post hoc test showed no significant difference between 1 week and 1 month in TiO2NPs modified type in all storage media.
4. DISCUSSION
This study aimed to evaluate the water sorption/solubility of glass ionomer cement incorporating titanium dioxide nanoparticles (TiO2NPs) in different storage solutions over time. Selection of the most suitable restorative material is important for the longevity of the restoration. The popularity of glass ionomer cement types is related to their properties, such as biocompatibility, the release of fluoride, and prevention of caries.
On the other hand, one of their main disadvantages; it can easily draw water into their structures. To increase the clinical success rate of restorations, it was recommended to investigate water sorption and solubility levels of restorative materials. Hydrolysis of the matrix of glass ionomer can occur by its water sorption; this fact leads to matrix deterioration over time, loss of the integrity of restoration margins, and surface characteristics [18].
For these reasons, many modifications have been made over the years in an attempt to improve the physical and mechanical properties of these materials. One approach is to incorporate nanoparticles as fillers [19]. Recently, studies showed that TiO2NPs have broad advantages in restorative dentistry due to their biocompatibility, remineralizing potential [20], and anti-caries effect [21]. TiO2NPs were shown to have a successful reinforcement of glass ionomer cement types [9].
The concentration of TiO2NPs used in the current study was chosen to be 10 wt % according to a previous study by Gjorgievska et al.(2020), which documented that blending GICs at 10 wt% of TiO2NPs was a successful formulation for improving the mechanical properties of conventional glass ionomers [22].
The sample size is one of the effective factors for the diffusion of water into the cement matrix. Smaller specimen size decreases the matrix stability. In a previous study, the sample dimension was chosen to be 10-15 mm in diameter and 1-4 mm in thickness [23]. Specimens diameter used in the current study was prepared as 10 mm, and the thickness was selected as 2 mm, according to Ozveren et al. [24].
Concerning oral hygiene measures, the use of mouthwashes is recommended complementing the effectiveness of toothbrushes and dental floss. Their chemical composition is based on different components such as; water, antimicrobial agents, salts, preservatives, alcohol, and hydrogen peroxide [25].
In the current study, different types of mouthwashes have been used to investigate their effects on water adsorption and solubility of glass ionomer cement. Residence time is known to affect the water adsorption and solubility levels of the material [26], so the residence times used in the current study were chosen to be 24 hours, 1 week, and 1 month.
The method used to test the adsorption and solubility in this study is a modification of section 7.12 of ISO 4049. The test requires that specimens be first placed in a desiccator immediately after curing and removal from the mold. In this study, section 7.12 was modified following Subramaniam et al. by placing specimens in the storage solutions immediately after preparation. The rationale behind this modification is to ensure that glass ionomer samples are not dehydrated immediately after fabrication, as it might affect their solubility and sorption results due to damage [27].
To determine the water absorption and solubility of the materials, different formulas have been used in different studies [28, 29].
All these methodological differences (sample size, storage solution, storage time, formulation of the water sorption, and solubility may lead to inconsistent results in the literature. We used the formulas used in a previous study by Singer et al. (2020) [18]. This evaluation was designed to assess the stability of moisture content in glass ionomer materials as to establish optimal conditions; the samples did not receive a protective coating to allow adequate exposure to moisture.
Regarding the effect of incorporating TiO2NPs into glass ionomers, results showed that the mean sorption and solubility rate of conventional glass ionomer was higher than that of TiO2NPs modified type, thus the first null hypothesis was rejected.
The improvements of both water sorption and solubility by incorporating TiO2NPs might be attributed to numerous explanations such as in conventional GIC, water sorption occurs primarily within the matrix, which leads to hydrolysis of the matrix and deterioration of the cement over time. On the other hand, nanofillers are water-insoluble so the supplementation of metal oxide to the matrix may decline its solubility.
This is in agreement with Dehis et al. (2018) [30], who documented a statistically significant reduction in water sorption and solubility of heat-cured and microwave-cured acrylic resin denture bases modified with TiO2NPs, which was comparable to the unmodified type.
This is also supported by a previous study by Subramaniam et al. (2015), which recorded a lower water solubility in glass carbomer-containing nanofillers when compared to conventional glass ionomers [27].
As for the effect of storage media on water sorption, both materials were associated with statistically significant differences between all storage solutions at 24hrs and 1 week, with similar effects after 1 month. Thus the second null hypothesis was accepted; the highest % of sorption was associated with alcoholic rinse at 24hrs. This could be explained by the fact that alcoholic content accelerates the early water diffusion of water.
On the other hand, artificial saliva showed the highest % of sorption at 1 week than other mouthwashes for both materials. This may be related to the change in the pH of the solutions over time, which may affect the rate of water diffusion. This finding can be confirmed by the stability of diffusion after 1 month of storage in all storage media.
The current findings are in contrast to those of Ozveren et al. (2021), who documented similar adsorption of high viscosity types of glass ionomer at 24h in all ambient solutions [24]. This difference may be related to the different types of ingredients and glass ionomer, and mouthwashes used in their study compared to the current study.
Regarding the effect of storage solution on the percent solubility, conventional glass ionomer showed a higher percent solubility in alcohol-free mouthwash at 24hrs compared to artificial saliva, which recorded the lowest solubility rate, with no significant difference between alcohol-free and alcohol type. On the other hand, a similar solubility rate was recorded in different storage media after 1 week and 1 month, thus the second null hypothesis was accepted. This fact may be explained by the fact that mouthwashes reduce the oral pH, which may accelerate the dissolution of the materials at the early storage time.
The current findings follow Ozveren et al. (2021), who documented lower solubility with high viscosity glass ionomer in artificial saliva when compared to alcohol and alcohol-free mouthwash at 24 hours [24].
On the other hand, TiO2NPs modified glass ionomer exhibited similar solubility in all storage solutions at 24hrs and 1 week and significantly higher solubility in alcohol-free mouthwash after 1 month. This finding could be explained by the structures of these materials, which are more resistant to the chemical components of the mouthwashes during early storage than conventional glass ionomer.
Regarding the effect of time on water uptake rate, results showed a statistically significant decrease for both materials when stored in artificial saliva and alcohol, similarly for TiO2NPs modified type when stored in alcohol-free. The highest % of sorption was recorded at 24hrs. Thus the third null hypothesis was accepted. These results can be attributed to the chemical composition of glass ionomer, in addition to the sensitivity of the hydrophilic matrix of the set cement to moisture within the first 24hrs.
This is consistent with the results by Elkafrawy et al. (2013), who recorded the highest sorption rates for different types of glass ionomer after 24hrs of storage in different mouthwashes and a decrease in sorption rate after 1 week of storage [31].
This also could be supported by Lima et al. (2018), who recorded the highest sorption rate with different types of glass ionomer at 24hrs, with a stabilization tendency for some materials at 7 and 14 days [32].
On the other hand, these findings are in contrast to Saves et al. (2019), the authors reported a time-dependent increase in the sorption rate of different types of glass ionomer [33]. The diversity of the results is due to the differences in methodology and composition such as materials and the storage media used in their study compared to the current study.
At the same time, water stability in the current study was not increased. This fact can be verified by the results of increased solubility of both materials during all evaluation periods, possibly due to the hydrolytic degradation of their surface.
Regarding the effect of time on solubility (%), results showed a gradual statistically significant increase in solubility (%) over time for conventional GIC stored in artificial saliva, similarly for TiO2NPs types stored in alcohol. Thus the third null hypothesis was accepted. This fact can be explained by the shrinkage of the specimen caused by the drying effect.
The current findings can be confirmed by a previous study that documented that, early protection of glass ionomer cement from hydration and dehydration using surface coating, increase the clinical success of the restorations. The surface coating reduces the early moisture exposure, advanced water absorption, and drying, leading to shrinkage and cracks of specimens [34].
Within the limitation of the current study, similar methodologies investigating the sorption and solubility behavior of the tested materials with a correlation to their mechanical performance are recommended. Different types of alcohol-containing mouthwashes, different PH media, and other different components of the mouthwash such as antimicrobial agents, salts, and preservatives need further evaluation on the sorption and solubility behavior of the cement. Clinical studies and further long-term studies must be conducted to confirm the results.
CONCLUSIONS
Based on the findings and the limitations of the current study, the following conclusions can be drawn:
- 1-TiO2 nanoparticles may improve water sorption and solubility of conventional cement.
- 2- Sorption and solubility percent of the tested materials were similar between different storage media at some time intervals.
- 3- The highest sorption rate occurred in the first 24hrs for both materials with a stabilization tendency for the modified GIC after 1 month in all storage solutions.
LIST OF ABBREVIATIONS
| GICs | = Glass Ionomer Cements |
| TiCl4 | = Titanium Tetrachloride |
| TiO2NP | = Titanium Dioxide Nanoparticles |
| SPSS | = Statistical Package for Social Sciences |
| ANOVA | = Analysis Of Variance |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


