All published articles of this journal are available on ScienceDirect.
Whitening Optical Effect of New Chewing Gums
Abstract
Background:
Today, many treatments are available to enhance the color of teeth, but they require a few days to weeks to deliver this effect.
Objective:
To evaluate the instantaneous optical whitening effect of two new sugar-free chewing gums and one dentifrice of proven efficacy versus one placebo chewing gum.
Methods:
This was a single-blind, parallel trial. 424 participants entered the study and were randomly allocated to four groups. They received a personal silicone mask with a calibrated hole at one upper incisor to apply a dental colorimeter. The tools tested were chewing-gum containing indigotine (E132-FD&C Blue 2) and spirulina, chewing-gum containing only spirulina, chewing-gum placebo, and dentifrice containing Blue Covarine. Vita® Easyshade scored the tooth color, and the WIO and WID whiteness indexes were calculated. For chewing gums, the color of the teeth was scored before the assumption and after 2’30” of mastication. For dentifrice, participants brushed for 1’30”, then rinsed, and the color was scored before brushing and after 2’30”. Statistical analysis was performed by ANOVA and Tukey post-hoc.
Results:
The two experimental whitening chewing gums and the dentifrice significantly increased the WIO and the WID indexes from the baselines (p<0.001). Moreover, their effects were statistically greater than those reported for the placebo chewing gum (p<0.05) but not significatively different among them (p=NS).
Conclusion:
All the tested whitening tools showed an instant optical whitening perception. Further studies are required to assess the intensity of the effect over a prolonged time to meet the people's request for fast whitening tools.
The clinical trial registration no. is PVM-2020-01.
1. INTRODUCTION
The wish for whiter teeth is often the main request of most people and a strong driver for the growth of the tooth whitening market. Therefore, tooth color is a popular topic among dentists and common people who wish to improve their smiles. It was reported that around 20% of the UK adult population were unhappy with their tooth color; in the USA, about 34%, and in an urban study population in China, 53% [1-3]. The perception of a tooth's color is determined by the combined effects of its intrinsic color and the presence of extrinsic stains [4-6]. Today, many treatments and materials are available to enhance the color of teeth as whitening dentifrices, professional cleaning and hygiene procedures, and bleaching agents to be applied in-office and at home [5-8]. Indeed, many consumer goods which claim to whiten teeth either by tooth bleaching or by removing or controlling extrinsic stains are available over the counter. However, they require a few days to weeks to deliver the suggested effect [7-9]. Collins et al. added blue covarine to a silica toothpaste to achieve an instant optical whitening effect by using dentifrice [7]. The delivery of this pigment after one single use of the dentifrice caused a blue shift in the yellow-blue color axis (CIE b*), which increased whiteness perception [6, 9]. Joiner et al. showed, in vitro, that blue covarine is deposited and retained on pellicle-coated tooth surfaces, causing a color shift that results in a perception of tooth whiteness [10]. In a recent trial, Tao et al. showed that two dentifrices containing blue covarine or a combination of blue covarine and FD&C Blue No. 1 (E133-Brilliant Blue) gave a statistically significant improvement in tooth whiteness immediately after a single brushing both in vitro and in vivo [11]. The clinical studies of Tao et al. and Collins et al. support the instantaneous whitening effect of blue covarine added dentifrice, meaning that the effect was found after a single use of the whitening dentifrice versus the daily use for several weeks of other formulae [7, 11]. Instead, a recent similar trial conducted with a different protocol by Schlafer et al. did not show a clinically relevant increase in tooth whiteness after a single brushing with a blue covarine containing dentifrice [12].
Chewing gum is very popular worldwide. It was proved efficient and a good vehicle for delivering many functional ingredients, some of which are also common with dentifrice [13, 14]. Therefore, a chewing gum added with pigments could have a similar whitening effect described with a dentifrice and be more practical to use outside, especially at social events.
The objective of this trial was to evaluate the instantaneous optical whitening effect of two new chewing gums and one dentifrice of proven efficacy versus one placebo chewing gum and to detect any possible side effects. The Null Hypothesis (HO) was that there was no significant difference in the whitening effect between the two sugar-free test chewing gums and the placebo chewing gum (negative control) or the dentifrice (positive control) after a 2’30” period.
2. MATERIALS AND METHODS
This was a single-blind, parallel randomized trial that involved two whitening sugar-free chewing gums, one clinically proven whitening dentifrice, and one placebo sugar-free chewing gum. Before qualification, all potential subjects signed a written informed consent and were examined using inclusion and exclusion criteria and conducting an oral hard and soft tissues screening inspection. Those recruited for the trial were checked for a dental impression to make their customized mask used at the test visit. The study population had to be between 18 and 60 years old, and the recruitment occurred until a minimum of 424 subjects had been pre-qualified to participate. They were allocated to four groups of 106 persons, each using a random table generated by the statistic software. To enter the trial, participants were required to read and sign the informed consent and other necessary paperwork before initiation of study procedures, be in good general health based on medical and dental history, and have a reasonably functional dentition with a minimum of 20 natural teeth (8 of which are posterior teeth, 4 of which are upper incisors), have all four sound upper incisors without any vestibular restoration, chew gum at least on an occasional basis, be willing to participate in the study, able to follow the study directions, and willing to return for all specified visits at their appointed time and be willing to postpone all elective dental procedures until the study was completed. To be included in this study, each potential subject had no advanced periodontal disease, five or more grossly decayed, untreated dental sites (cavities), pathologic lesions of the oral cavity (suspicious or confirmed), full or partial dentures, or fixed orthodontic appliances, except for a fixed, lower lingual retainer, diagnosis of xerostomia or impaired/decreased salivary function, a medical history indicating that the subject is pregnant or currently breastfeeding. Each subject had no simultaneous participation in another clinical research study, a history of allergy or intolerance to gum ingredients, including (but not limited to) soy, phenylalanine, low-calorie artificial sweeteners, artificial colors or flavors, mint, peppermint, spearmint, milk proteins, or other ingredients, have a serious medical illness or disorder, e.g., immune-compromised, AIDS, etc., that would be unduly affected by participation in this study, a history of the temporomandibular joint disorder (TMJ). No subject was excluded because of his/her teeth color at baseline. However, the teeth to be examined had to be free of extrinsic stain and free of intrinsic stain. All eligible participants were required to avoid any oral hygiene and consuming food or drinks for two hours before the test except for water which will be allowed/encouraged up to 1 hour before each subject’s scheduled appointment time. They received a plain dentifrice containing only sodium monofluorophosphate (Elmex®, Gaba, Colgate-Palmolive, PL-58-100 Świdnica) for three days before the study and for brushing two hours before the test. All enrolled participants were asked to be available for an upper full-arch silicone impression. A dentist made this and immediately sent it to a dental laboratory for modifications to realize an individual mask with a calibrated hole approximately at the center of the buccal surface of one upper incisor to apply the tip of the Vita® Easyshade Advance 4.0 device to score the color with CIELAB color space coordinates in the same condition for all the measurements. This method was investigated and validated by Perra et al. [15]. The L*a* b* absolute values were influenced by the color of the mask due to reflection from the material, but this was not the focus of this study which was planned to assess the optical whitening effect as captured by the variations of the optical coordinates and following indexes and not to score the real color as it is the case in dental practice, especially for prosthodontic procedures. The use of the Vita® Easyshade Advance 4.0 device for assessing whitening procedures was recently supported by other researchers [12, 16, 17].
The CIELAB is a well-known universal method based on three coordinates (L a b) to define one exact color among the infinity of possible colors of an item, and therefore it is used in industrial procedures. In the past, authors developed two whitening indexes (WIO and WID) calculated from the L*a*b* values and published papers to validate them for evaluating dental whitening procedures [18]. Many authors followed their suggestions, used the two indexes, and published some papers [7, 11, 12]. Therefore, the literature supports using WIO and WID as parametric indexes with a normal distribution in the population. However, it was performed the Kolmogorov-Smirnov to assess their normal distribution in each group again. During each visit, the operator performed a comprehensive oral examination consisting of an evaluation of oral hard and soft tissue structures. Oral hard tissues examination was performed with a dental mirror to examine teeth and bony structures, while oral soft tissues examination was performed by examining the mouth and pharynx, including lips, tongue, the floor of the mouth, palate, gingiva, alveolar and buccal mucosa, oropharynx, tonsils, uvula, and salivary glands using palpation techniques and visualization. Visualization and bimanual palpation were performed for extraoral examination of the head and neck regions. Any abnormalities noted might constitute a reason for exclusion or dismissal from the study. In case an oral-related adverse event was observed during a testing visit, the study dentist decided whether the event was related to the testing chewing gum or the dentifrice following his/her examination of the oral cavity and documented his/her findings.
2.1. Tools
Whitening chewing gum 1 was coated, 1.4g per piece, added with indigotin (E132-FD&C Blue 2) and spirulina. The total amount of spirulina was 1200 ppm of the finished product, and the total amount of E132 was 200 ppm. The type of spirulina was characterized by color strength 1.260-1.540; E132 was complying with purity criteria reported in current EU food regulations. Spirulina was concentrated only in the center and the E132 in the coating.
Whitening chewing gum 2 was coated, 1g per piece, added with spirulina; the total amount of spirulina was 1700 ppm of the finished product distributed in the center (800 ppm) and the coating (900 ppm). The type of spirulina was characterized by a color strength of 1.260-1.540.
Placebo chewing gum was coated, 1.4g per piece, without any color, pigment, dye, or other whitening ingredients.
Dentifrice was the Mentadent White Now® (Unilever, London, UK) toothpaste containing Blue Covarine CI 74160. HPLC analysis showed that it contained about 0.03% of blue covarine; therefore, 1.5g of toothpaste had 0.45mg of Blue Covarine.
All the chewing gums investigated were sugar-free and provided by the manufacturer (Perfetti Van Melle S.p.A., Lainate, MI, Italy). Mentadent White Now® dentifrice was purchased from the market. The brushing on the day of the experiment was performed with a brand-new standardized toothbrush (SmileGoods®, Practicon, Inc., Greenville, NC).
2.2. Protocol
The color of the tooth pre- and post-treatment was scored by the portable device Vita® Easyshade Advance 4.0 in the CIELAB color space according to methods shown in the previous studies [12, 15-17]. The tip of the device was positioned on the tooth surface into the guide of the silicone customized mask. A new reset/setting of the device was performed at the end of each participant test according to the manufacturer's instruction and employing the specific white tile. All the measurements were performed using the protective pellicle for the tip provided by the manufacturer and following the instrument instructions by the same operator in a dental office provided with artificial illumination [17]. Mastication of chewing gums or use of dentifrice were performed in a different room of the same dental office. Therefore, the operator was blinded by the tool, but the participant was not. In the group with the dentifrice, the color was measured before brushing, then participants were requested to brush their teeth with the dentifrice, in an amount covering the toothbrush head (1.5g), for 1’30” and to rinse with 5cc of water for 5” at the end of brushing as reported in previously published trials [11]. The color was scored twice: before brushing and after 2’30”, which was the total time needed to perform all the operations, comprising going to the basin, brushing, rinsing with the indicated procedure and other necessary operations, between the pre-treatment and the post-treatment, reads. Likewise, in the three groups receiving the different types of chewing gums, the color of teeth was scored twice: before the assumption of one piece of the selected chewing gum and after 2’30” of mastication. The mastication was supervised, but volunteers were encouraged to chew at their normal pace. Each measure was scored as the mean of a triple measurement based on the CIE for L*a*b* color space, and then the WIO and WID whiteness indexes were calculated [18-21]. Researchers developed WIO from the CIE whiteness formula, and it was found to give the best performance for predicting tooth whiteness based on their laboratory results [18]; additionally, it was also calculated the WID index according to published equations [19-21].
2.3. Statistical Analysis
All data were analyzed with SPSS software (IBM, Armonk, NY, USA) for mean, SD, SE and variance. The differences in whitening were analyzed by L*, a*, b*, WIO, and WID between baseline and after 2’30” with the paired t-test. Statistical analysis among the groups was performed by repeated measures ANOVA with Tukey post-hoc test. The Kolmogorov-Smirnov test assessed the normal distribution of the whitening indexes used.
2.4. Power and Sample Size
There were no published trials with chewing gums and their optical whitening effect on teeth. One similar study with a dentifrice containing blue covarine was performed by Collins et al. but in a crossover design; however, from the data reported in this trial, if we set α=0,05 and the power at 80%, the minimum number of teeth to test with a parallel groups design was 106, therefore, for this study, each group composition was set at 106 subjects [7].
2.5. Ethical Issue
This study followed ethical principles in the Declaration of Helsinki and approximate Good Clinical Practice guidelines [22]. The chewing gums used in this trial were without active pharmaceutical agents and constituted by authorized food ingredients and additives; therefore, they were considered a common food and administrated in a common dosage. The dentifrice used in this trial was available on the counter. This study was approved by the ethical committee “U.S. Investigational Review Board, Inc.”, with IRB Number U.S. IRB2020PVM/01, on April 21, 2020. This trial was conducted according to the local guidelines for COVID prevention.
3. RESULTS
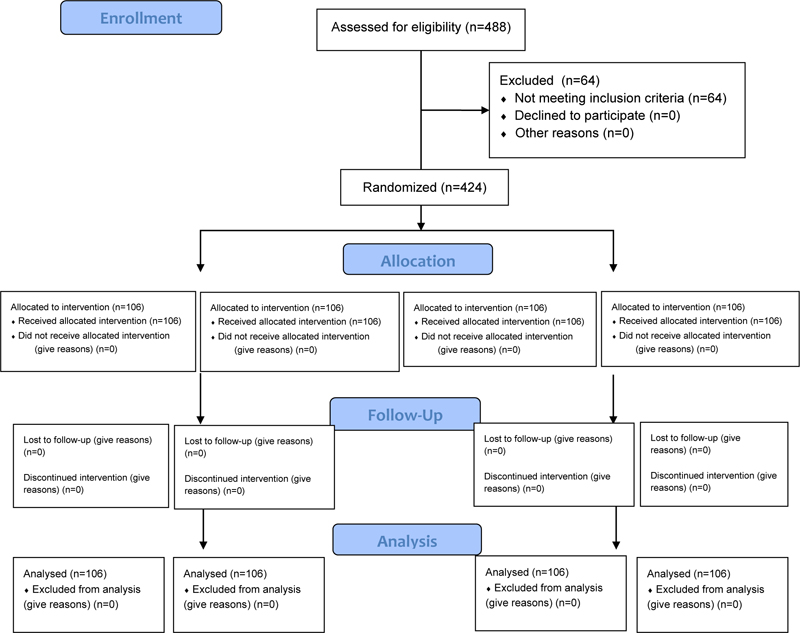
From a pool of 488 subjects examined, 424 eligible individuals joined this trial. All the participants enrolled completed the study. Therefore, they were 424 between 19 and 58 years old (mean 35.2±11.4), 238 females, and 186 males (Fig. 1). The four groups of 106 each were composed of males and females in similar proportions as reported in Table 1. The Kolmogorov-Smirnov test assessed each group's normal distribution for WIO and WID. The outcome of the trial was reported in terms of differences post and pre-treatment for all the tested parameters in each group. The WIO and WID differences pre-post treatment were statistically significant (p<0.001) within the group for each treatment except for the placebo chewing gum. The results and the statistical differences intra-group and among the groups were shown and summarized in Table 2. Repeated measures ANOVA and post-hoc test (Tukey) showed that all the three whitening tools, both whitening chewing gums and the dentifrice, increased the WIO and the WID significatively more than the placebo chewing gum (p<0.05), but they were not statistically different among them; therefore the null hypothesis (HO) was rejected. The acceptance of all chewing gums and dentifrice was high, and no subjects reported any related problems or side effects.
| Group | Age | Sex |
|---|---|---|
| E132 + Spirulina Chewing Gum | 34.9±11.6 | F60 M46 |
| Spirulina Chewing Gum | 36.3±11.0 | F62 M44 |
| Placebo Chewing Gum | 33.9±11.1 | F61 M45 |
| Dentifrice White Now® | 35.8±11.8 | F55 M51 |
| Group | ∆L | ∆a | ∆b | ∆WIO | ∆WID |
|---|---|---|---|---|---|
| E132 + Spirulina Chewing Gum | -0.21±1.87a | -0.20±0.88a* | -0.74±1.05a** | 1.82±2.56a** | 1.15 ± 4.07a** |
| Spirulina Chewing Gum |
-0.01±1.11a | -0.20±0.68a* | -0.49±0.77ac** | 1.56±1.99a** | 0.99±2.90a** |
| Placebo Chewing Gum |
0.03±1.95a | 0.07±0.46b | -0.03±1.00b | 0.03±1.92b | -0.10±1.33b |
| Dentifrice White Now® |
0.24±1.87a | -0.07±0.68ab | -0.37±1.24bc* | 1.54±3.40a** | 0.69±3.02a** |
Inter-groups: p<0.05 shown by a different letter (Repeated measures ANOVA with Tukey post hoc test).
4. DISCUSSION
In the present study, it was employed as a principal variable, the WIO, a specific index developed to assess the perception of tooth whiteness effect in dentistry and its change over time [18]. The same index was also used in previous studies regarding the optical whitening effect of dentifrices containing blue covarine or a mixture of blue covarine and FD&C Blue No.1 (E133-Brilliant Blue) [7, 11]. In the trial conducted by Collins et al. with a blue covarine dentifrice, the ΔWIO was 1.14 (p<0.05) for the test dentifrice and 0.08 (p=NS) for the negative control dentifrice [7]. In the research performed by Tao et al., the ΔWIO were 0.68 and 5.62 with dentifrice containing respectively blue covarine or a mixture of blue covarine and FD&C Blue No.1 when measured in vitro and, respectively, 2.28 (p<0.001) and 5.51 (p<0.001) when measured in vivo [11]. The present trial showed the same instant optical whitening effect of all the tested tools containing blue coloring agents, which were more effective than the placebo chewing gum without any coloring or whitening agent. Interestingly, the results were statistically comparable irrespectively to the classification of the coloring agents as soluble coloring dyes (E132-FD&C Blue 2) or insoluble pigments (blue covarine) or coloring foodstuff (spirulina), while previous studies suggested a superior effect exerted by insoluble pigments over soluble dies [10]. The dentifrice was used as the positive control in this study, and the chewing gums containing food-grade colors, were instead effective towards the baseline and the negative control chewing gum after one single application, calling for an instant tooth whitening effect as already reported in the previous publications [7, 11]. However, this is the first clinical study reporting a whitening effect in terms of ΔWIO exerted by chewing gum; for this reason, the duration of the chewing time was modeled on the 1’30” of brushing reported by Collins et al. and confirmed by Tao et al. with the addition of the time needed for the controlled rinse and other operations needed in between the pre-and post-reading, thus reaching an overall 2’30” between the pre-and post-treatment reading; therefore the same time was then applied to chewing duration as well [7, 11]. Considering that the survey by Hearty et al. reports that 90% of adolescents and adults declare chewing a gum piece for more than five minutes, we believe that the effect is registered within the usual time of consumption of chewing gum [23]. In the present study, we found that chewing gums of two different formats (1g and 1.4g per piece) have a whitening effect that is not distinguishable from each other or the positive control dentifrice; therefore, we suppose that any effect due to the weight or to the type of product is secondary respect to the amount in pigments or dyes when the time of consumption is comparable. In the present study, the commercial dentifrice used as positive control and containing blue covarine scored an average ΔWIO of 1.54. This value is between the average ΔWIO values found in previous studies with covarine-based toothpaste [7, 11]. Thus, the present method, employing the system Vita® Easyshade Advance 4.0 and silicone masks, seemed to be able to capture within a clinical trial the same optical whitening effect as the DIS arrangement used in the previous studies [7, 11, 19]. The standard errors (SE) were calculated for the ΔWIO index from ±0.19 to ±0.31, the latter for the White Now® dentifrice. Collins et al. found with the same index a SE of ±0.30 and ±0.31 for the blue covarine dentifrice and the negative control, respectively [7]. Tao et al. reported a SE in the range of ±0.32 to ±0.41 in the in vivo part of the study [11]. Therefore, the data dispersion was probably comparable between the present study and the previous ones. The objective assessment of tooth whitening through instrumental methods, especially versus visual procedures, gained increased attention and the VITA® Easyshade instrument was already used in several clinical studies to measure the color coordinates in vivo [12, 16, 24-28]. The method of the present clinical trial, which employed the Vita® Easyshade Advance 4.0 device, allowed the comparison of clinical data obtained in the present study with the ones from published literature, supporting that this method could be used to detect the optical whitening effect of chewing gum, dentifrice, or other whitening devices in vivo [7, 11, 15]. The use of the silicone mask surely influenced the real color coordinates measured for the tooth tested, but it cannot ever influence the differences reported between the two measures (post- and pre-whitening), which were taken with the same mask and the same procedure [15]. The positive shift in the ∆WIO was measured for all the test gums, and the dentifrice was positively influenced by the negative Δb* shift (blue shift). The Δb* found for the White Now® dentifrice in the present study (-0.37) is like the one found by Collins et al. (-0.36) [7]. Study gum 2 caused a similar Δb* shift, while study gum 1 was statistically superior (Δb*=-0.74). The blue shift is a major determinant for the perception of the whitening effect after the use of bleaching products and may contribute to a perception of whitening after the single use of the three products with coloring agents tested in this study [7, 9]. However, the same ΔWIO in absolute value can be more or less perceptible according to the main trigger contributing to the final index being L*, a*, b*, or combinations of these parameters, making it difficult to establish universal thresholds [29]. For these reasons, this trial also calculated the new WID index, which was expressly developed to assess whitening in dentistry and reported to correlate to the visual observation of whitening better than the other indexes, while it was comparable to the WIO index for accuracy in correlation [30]. Also, for the WID index, the differences post- to pre- treatment were statistically similar among the treatments with the two study gums (ΔWID=0.99 and 1.15) and the dentifrice (ΔWID=0.69) and statistically different from the placebo chewing gum (ΔWID=-0.10). In particular, the WID index was found to have perceptual thresholds of 0.46 units for experts and 0.94 for inexperienced observers, below those observed in the present study [21]. In summary, the current study registered an objective shift in three parameters Δb*, ΔWIO, and ΔWID, which may contribute to the perception of tooth whitening [10, 21]. The parameters of this study were, however, determined in controlled conditions of light, while real-life perception can be influenced by the available type and intensity of the light source, the context of contrasting colors from the gums, lips, and skin, and the angle and distance of observation. All these influencing factors will require additional research in the future. This was the first clinical study with a parallel-group design to compare the instant whitening effect of chewing gum with a dentifrice whose efficacy is supported by scientific literature. No adverse effect was reported due to the assumption of chewing gum or dentifrice use. The single use of all the three whitening tools, whitening chewing gums and dentifrice, increased the WIO and the WID indexes significatively more than the placebo chewing gum. There were no statistical differences among the optical whitening effect found with the use of a dentifrice purchased on the market and reporting instant optical white claims and both the whitening chewing gums; therefore, the chewing gum containing E132 and spirulina or the chewing gum containing only spirulina showed a similar optical whitening effect as the White Now® dentifrice. Due to the limitations to comparing dentifrice to chewing gums which results in a no-blinding test for participants, and for the time of chewing, which had to be like dentifrice, it must be recommended to plan further studies to confirm these observations, especially over a longer time.
CONCLUSION
All the tested whitening tools showed a statistically significant increase in instant optical whitening perception as approximated by the WIO and the WID indexes versus the placebo chewing gum. Therefore, both the tested whitening chewing gums showed a similar optical whitening effect as the White Now® dentifrice. Further studies are required to assess the intensity of the effect over a prolonged time to meet the people's request for fast whitening tools.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the ethical committee “U.S. Investigational Review Board, Inc.”, with IRB Number U.S. IRB2020PVM/01, April 21, 2020.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013. This trial was conducted according to the local guidelines for COVID prevention.
CONSENT FOR PUBLICATION
Before qualification, all potential subjects signed a written informed consent and were examined using inclusion and exclusion criteria and conducting an oral hard and soft tissues screening inspection.
STANDARDS OF REPORTING
CONSORT guideline has been followed.
AVAILABILITY OF DATA AND MATERIALS
The author's data associated with this paper can be accessed upon request.
FUNDING
Perfetti Van Melle S.p.A. sponsored this research and the Ethical Committee fees.
CONFLICT OF INTEREST
The authors are scientific consultants of Perfetti Van Melle S.p.A, and they declare no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


