All published articles of this journal are available on ScienceDirect.
Current Trends in Anticancer Drug Delivery System for Oral Cancer- A PRISMA complaint Systematic Review
Abstract
Background:
Oral cancer is a deadly disease affecting worldwide. Despite developments of conventional cancer therapy, there has been little improvement in the survival rates. This culminated in the evolution of a targeted. New Drug Delivery System, discovering novel objectives for successful drug delivery and synergistic combination of anticancer agents to minimize side effects.
Objective:
The main focus was on understanding the various aspects of different targeted drug delivery vehicles used in the treatment of oral cancer including advantages, disadvantages, and future perspectives.
Materials and Methods:
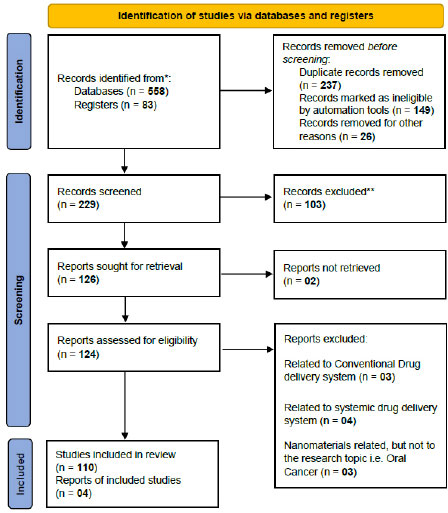
A literature search was accomplished from 2005 to 2020 via Google scholar. PubMed, EBSCO, Embase, and Scopus databases along with Clinical trials registries using the terms oral buccal thin films, Hyperthermia and Thermoablation, Intra-tumoral, Photodynamic, Immunotherapy, photothermal, and ultrasound therapy in oral cancer. The articles were scrutinized and those which were not relevant to our search were omitted. Clinical trials on targeted drug delivery systems for Oral Cancer being conducted or completed around the world from various registries of clinical trials have also been searched out and the findings were tabulated in the end. The PRISMA 2020 guidelines were followed.
Results:
The treatment of oral squamous cell carcinoma (OSCC) mostly depends upon the location, type, and stage of the tumor. Vivid targeted drug delivery systems are being used in the therapeutic interventions of oral cancer as they aim for specific target site delivery and are the most appropriate treatment. Active Pharmacological Ingredient (API) is taken to the targeting site, sparing non-target organs or cells, triggering selective and efficient localization, thereby maximizing the therapeutic index with minimizing toxicity. The successful targeted drug delivery system works on four principles i.e. Retain, Evade, Target and Release, which means loading of sufficient drug into a suitable drug carrier, does not affect body secretions, long duration in circulation, reaching the targeted site and, drug release within the time for effective functioning of the drug. All techniques described in this paper have proven to show effective results.
Conclusion:
Oral Cancer is an emerging public health problem worldwide. Various conventional therapies are used for treating oral cancer, but they enclose variable degrees of side effects both on the body as well as the cellular microenvironment. With advanced technology, many other aids have been introduced in the field of oncology to treat oral cancer with minimal side effects. All techniques described in this paper have proven to show effective results in the therapeutic interventions of oral cancer. Moreover, they can be used even in combination with conventional drug therapy to show beneficial outcomes. Several clinical trials are being conducted and completed in this aspect to investigate definite results of these therapies, yet robust research is needed for further confirmation.
1. INTRODUCTION
Oral cancer is an emerging public health problem in Asia and is among the foremost devastating diseases. As per the GLOBOCAN 2018 report, the prevalence and mortality for various types of cancers in 185 countries have increased with 18.1 million new cases and approximately 9.6 million deaths [1]. The main treatment for oral cancer includes chemotherapy, surgery, and/or radiation therapy. Chemotherapeutic agents in clinical use worldwide often have serious toxic effects on the cells and tissues, resulting in various kinds of adverse effects on the body. Also, the development of resistance to such drugs decreases the actual clinical benefits. Despite developments in conventional cancer therapy, there has been little improvement in the survival rates. This has culminated in the evolution of a systematic NDDS (New Drug Delivery System) for discovering novel objectives for successful drug delivery and synergistic combination of anticancer agents to minimize the drug concentration, reduce toxicity, increase drug effectiveness, and boost the survival rate. Controlled drug delivery technology has improved dramatically in recent decades, resulting in the creation of a variety of therapeutic formulations that improve patient compliance and convenience. Targeted medicines have been developed as a result of a better knowledge of some of these changes with the hope that they will eliminate malignant cells without damaging healthy cells. The term “targeted drug delivery” refers to the predominance of drug accumulation within a target zone that is independent of the drug administration method and route. Targeted therapy or targeted medicine, on the other hand, refers to the molecular interaction between a medication and its receptor [2-4]. Retain, evade, target, and release are four fundamental characteristics of effective targeted medication delivery systems [5].
The treatment of oral squamous cell carcinoma (OSCC) mostly depends on the location, type, and stage of the tumor. The unsolicited effects of chemotherapy can be reduced by nanotechnology and targeted drug delivery systems. Targeted therapy aims for specific target site delivery and is the most appropriate treatment for oral cancer [6]. Moreover, it can be used even in combination with conventional drug therapy to show beneficial outcomes. This systematic review article discusses different targeted drug delivery vehicles used in the treatment of oral cancer and their future perspectives.
2. METHODOLOGY
A planned systematic literature review of studies that proved the importance of different kinds of treatments for oral cancer other than conventional treatment, like chemotherapy, surgery, and radiotherapy, was conducted using online databases. Literature search, which was limited to English publication, was accomplished using Google Scholar, PubMed, EBSCO, Embase, Web of Science, and Scopus databases, along with Clinical trials registries using the terms oral buccal thin films, hyperthermia and thermoablation, intra-tumoral, photodynamic, immunotherapy, photothermal and ultrasoundtherapy in oral cancer. The scientific publications from the last 15 years, i.e., 2005 to 2020, were included. The articles were scrutinized, and those irrelevant to our search were omitted. Only publications focusing on the treatments of oral cancer other than conventional treatment, like chemotherapy, surgery, and radiotherapy, were eligible for inclusion. Additionally, data were collected by electronic and hand searching, cross-referencing along with the help of internet engines. Two independent investigators performed all the searches, including title and abstract screening; however, abstracts from conferences and commentaries were excluded. Articles discussing nanotechnology as another aid in conventional drug delivery have also been excluded. Any discrepancies were resolved through consensus. The main focus was on understanding the various aspects of different targeted drug delivery vehicles used in the treatment of oral cancer, including advantages, disadvantages, and future perspectives. We have attempted to search various clinical trials on these local and targeted drug delivery systems for oral cancer being conducted or completed around the world from various registries of clinical trials; the findings have been tabulated in the end. The clinical trials that were withdrawn were excluded. Fig. (1) shows the PRISMA 2020 flow chart describing the selection of the 114 articles after multiple phases of the screening process.
2.1. Local Drug Delivery and Drug Targeting
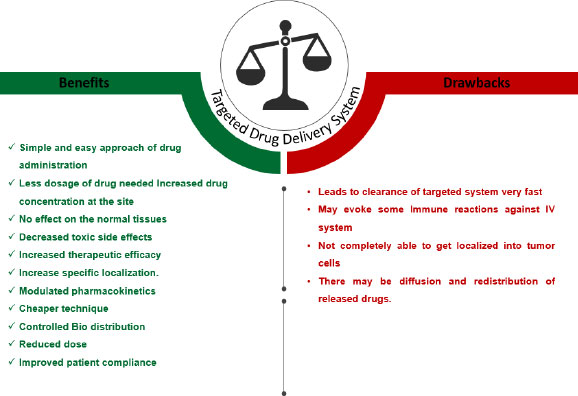
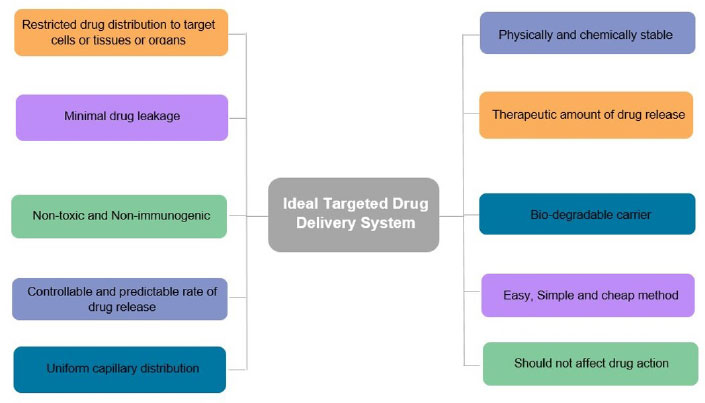
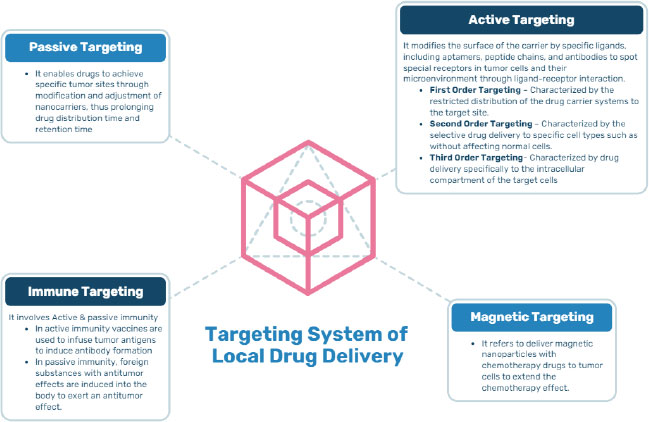
Targeted/Local drug delivery (TDD) is a strong and specific evolving method in cancer management where Active Pharmacological Ingredient (API) is taken to the targeting site, sparing non-target organs or cells, triggering selective and efficient localization, thereby maximizing the therapeutic index along with minimizing the toxicity. The drug is directly transported either within or in the proximity of the target through drug delivery vehicles that transport the drug. The successful targeted drug delivery system works on four principles, i.e., Retain, Evade, Target and Release, which means loading of sufficient drug into a suitable drug carrier, not affecting the body secretions, maintaining circulation for a long duration, reaching the targeted site, and releasing the drug within the appropriate time for effective functioning of the drug [7]. Local drug delivery is a type of drug delivery in which a drug is transported to the targeted site without affecting the normal body system as compared to conventional routes, thus preventing the side effects on healthy cells. This method has been shown to have beneficial effects on oral tissues in oral cancer, such as rapid oral mucosal turnover leading to self-repair, thus alleviating the adverse effects caused due to long-term exposure to anticancer drugs. Local drug delivery has many benefits and drawbacks, as summarized in Fig. (2). An ideal targeted drug delivery system must possess certain characteristics that make it efficient to apply for the technology represented in Fig. (3). There are different targeting systems for drug delivery as described in Fig. (4).
2.2. Various Methods of Local and Targeted Drug Delivery for Oral Cancer
2.2.1. Fast Dissolving Oral Thin Films
A modern delivery method called Fast-dissolving Drug Delivery System (FDDS) is being designed to amplify the shortcomings of traditional drug delivery. FDDS are of two main types, i.e., Oral Disintegrating Tablets (ODTs) and Oral Thin Films (OTFs); OTFs further comprise Mucoadhesive Buccal Films (MBFs) and Oro-Dispersible Films (ODFs). MBFs provide an effective site-specific drug. ODFs must possess a few unique characteristics, which include fast dissolution, adhesiveness to the mucosa, systemic drug administration, API (Active Pharmaceuticals Ingredient) swallowed with saliva, and GIT route of absorption. The system has a solid dosage in the form of strips, which, when kept in the mouth, dissolves quickly without even the use of water. Table 1 describes the basic constituents of OTF that are used with Active Pharmaceutical Component (API). OTF is very useful in ambulatory patients who report difficulty swallowing pills. Schiele et al. reported that of 1051 patients, about 37.4% of patients experienced difficulty swallowing medicines, and 1 in 3 such patients experienced vomiting, gagging, choking, or having tablets blocked in the throat [8]. OTFs treat a wide range of diseases, such as migraine, schizophrenia, opioid dependence, pain, nausea, and vomiting, and are now being used in oral potentially malignant lesions and oral cancer or as adjuvant therapy. In 2009, Shimoda H et al. indicated that dexamethasone-containing fast-dissolving OTF is used as an effective cancer chemotherapeutic agent [9]. Ramineni et al. studied the effect of mucoadhesive buccal films loaded with imiquimod for treating oral precancerous lesions, and concluded that it resulted in enhancement of drug release than other conventional forms [10]. In 2014, Costa et al. suggested chitosan-based mucoadhesive film along with photodynamic therapy to boost the retention of 10.0% of 5-ALA in tumor tissue, and found it to be a reliable aid in oral cancer therapy for local drug delivery [11]. Pawar and Kamat developed and evaluated the mouth dissolving film of the drug ‘ondansetron hydrochloride’ using taro gum and observed an improvement in its bioavailability, release, disintegration time, and tensile strength in comparison to the conventional methods [12]. Jin Bao Z et al. investigated the role of mucoadhesive patches loaded with methotrexate (MTX) liposomes in the local drug delivery system for oral cancer, and found it to exhibit suitable bio-adhesive properties and prolonged release time [13]. ETFs are an improved drug delivery system that offers many advantages over more traditional delivery methods. It has more advantages than disadvantages, as described in Table 2.
2.3. Hyperthermia and Thermoablation
For centuries, heat has been used for therapeutic purposes in the medical field. Human cells are capable of withstanding a wide range of temperatures. Generally, complete necrosis occurs almost instantaneously at temperatures <−40 °C to >60 °C [14]. Hyperthermia (HT) is a non-invasive technique where body tissue is exposed to high temperatures ranging from 410C-500C, whereas thermoablation includes temperatures above 500C [15]. The main objective of thermoablation in cancer is the removal of damaged tissue along with healthy margins by applying heat and electromagnetic waves. It involves some of the common methods, such as Radiofrequency electrical current (RF), Microwave frequency radiation (MW), Laser, Magnetic induction, Cryoablation, and High-intensity focused ultrasound (HIFU). In thermal ablation, the option of an effective heat delivery route to the tumor is a critical and challenging concern and is used with imaging [16]. The effects of hyperthermia depend on the duration of HT, temperature homogeneity in tissue, type of tissue type, and type of aid used [17-19]. The mechanism of local hyperthermia involves the direct cell-killing effects inducing apoptosis, DNS damage and reduction of perfusion, coordination effect enhancing radiation sensitivity and drug absorption, hypoxia, and immune modulation. For successful treatment of cancer, HT can be given in combination with conventional cancer treatment modalities, such as radiation and chemotherapy. Tumors become radiosensitizers in the presence of thermal stress, making them responsive to radiotherapy and improving cancer survival rates. The chemotherapeutic drugs can be loaded with nanoparticles via active or passive targeting. Brook et al. assessed the efficacy of RF ablation aided by computed tomography in the palliative therapy of 14 advanced malignancies of the head and neck that recur frequently with 27 applications; 100% success was observed in tumor targeting and 67% showed life improvement, while 11% of applications showed major complications, i.e., stroke, carotid blowout with death, and threatened carotid blowout with subsequent stroke [20]. Belfiore et al., in their retrospective study conducted from 2002-2014, evaluated 22 patients with unresectable HNC treated with thermal ablation and observed better survival rates, and concluded that this technique could prove to be a successful treatment for treating HNC locally [21]. Guenette J P et al. applied percutaneous image-guided cryoablation in 7 cases of head and neck tumor and found that no major complications were reported except for intra-procedural bradycardia in one case and nasopharyngeal abscess in another [22]. Mohan et al. studied the antitumor effectiveness of magnetic induction interstitial hyperthermia with a ferromagnetic needle for tongue cancer and found successful results. Eight patients with oral cancer were treated using hyperthermia combined with chemotherapy and showed complete response [23]. This method exhibits many benefits as compared to the traditional drug delivery system, but also involves a few limitations, as described in Table 2.
| Component | Types | Properties |
| Polymers | - Polyethylene oxide, Poly vinyl alcohol, Hydroxy-propyl methyl cellulose, Polyvinyl pyrrolidone, Pullulan, Chitosan, Sodium alginate, Gelatin, Hydroxypropyl cellulose, etc. | - Improve film-forming features. - Resistant to tearing. - Increase flexibility. |
| Mucoadhesive Polymers | - Polyox WSR Newer Second Generation Polymers, Hydrophilic Polymer, Lectins, Thiolate polymers, Novel Polymers, etc. | - Quickly adhere to the buccal mucosal membrane - Quickly hydrate - Non-poisonous - Non-irritant - Cheaper |
| Permeation Enhancers | - Polysorbate 80, Azone, Lauric Acid, Propylene Glycol, Aprotinin, etc. | - Increase fluidity of bilayer lipid wall. - Increase drug absorption |
| Sweeteners | - Glucose, Maltose, Sucrose, liquid glucose, dextrose, fructose etc. | - Increase palatability |
| Flavouring Agents | - Synthetic flavour oils, oleo resins, plants extracts - Flavour oils (Nutmeg, cinnamon, spearmint, peppermint, etc.) - Fruity flavours (citrus, cocoa, chocolate, coffee, vanilla, etc.) - Fruit essence (Pineapple, Apple, Cherry, Raspberry, etc.) |
- Acceptance of the formulation - Masking the taste |
| Saliva Stimulating Agent | - Lactic, Malic, Tartaric, Ascorbic, and Citric Acid | - Increases saliva production - Helps faster disintegration of formulation |
| Plasticizers | - Glycerol, Glycol (Propylene), Phthalate derivatives (Diethyl, Dimethyl, and Dibutyl phthalate), Low Mol. Weight Polyethylene Glycol, Citrate derivatives (Triethyl, Tributyl, Triacetin, Acetyl citrate, Castor oil, etc. | - Improve flexibility - Reduce bitterness. - Enhance the strength of the polymer. |
| Colouring Agents | - FDA-approved colouring agents are used | - For maintaining aesthetics |
| Methods | Advantages | Disadvantages |
| Fast Dissolving Oral Thin Films | - Prolong the drug residence time at the site of absorption - Enhance flexibility - Excellent accessibility - A simple way of drug administration - Patient compliance enhanced. - Rapid bioavailability and fast acting - Improved biopharmaceutical properties - Lead to direct entry into the circulatory system - Do not get affected by the acidic environment in the gastrointestinal tract. - Help to discover another route of drug administration. - Deliver a larger proportion of Active Pharmaceutical Ingredients per dose - Easy administration in trauma and unconscious patients, mental disorders, dysphagia, motion sickness and repeated emesis - Ease of transportation, storage and consumer handling |
- Drugs with irritating effects on oral mucosa cannot be administered - Limited drug load - Bitter taste - Testing OTF characteristics is a challenge - Dose uniformity is a technical challenge - Eating and drinking are restricted till OTF is applied - Require special packaging - Expensive - Involuntarily swallowing may lead to choking. - There may be a loss of drug suspension due to the swallowing of saliva. |
| Hyperthermia and Thermoablation | - Minimally invasive - Flexibility - Low cost - Cause apoptosis of cancer cells - Improve therapeutic efficacy - Cell-cycle independent cellular damage - Palliative treatment for untreatable malignancies |
- Principally work on the aid of imaging - Entire technique and treatment effects rely on effective imaging - Suitable heat delivery route to the tumor is required. - Care has to be taken to preserve vascular components to avoid damage |
| Intratumoral Therapy | - More drug concentrations at the tumor site. - Minimal toxicity - Helps in reducing local tumor size - Has shown to provide less complications leading to metastasis - Can lead to multiple needles to be injected with same needle without affecting drug administration. - Improves the results when local therapy is combined with surgical. - Provides easy accessibility of the primary site |
- Increased interstitial pressure leads to more risk of extravasation. - There is a risk of vascular collapse when the lesion approximates the region of large vessels. |
|
Photodynamic Therapy |
- Targets only cancer cells sparing normal cells - Can be delivered as an outpatient procedure - Surrounding margins of visible lesions incorporating borders of normal mucosa can conservatively be included in the target location - Unlike Radiotherapy, PDT can be repeated - Less risky - Less time taking - Inexpensive - Effects on tumor vascularity - May alter the immune response - Offers the potential for improved functional and cosmetic outcomes. - Can be used as an adjuvant in combination with surgery. - Reduces long-term morbidity - Maintains normal tissue functioning |
- Treats areas where light can reach. - Local skin reactions - Limited penetration of light leads to a very less depth of tumor destruction - Cannot completely replace other treatment modalities - Phototoxic effects, like erythema and edema, after the treatment, contact dermatitis - Immunosuppression - Mild to moderate pain at treatment sites - Patient is advised to avoid sun for a few weeks. |
|
Photothermal Therapy |
- The capability for deep tissue penetration - Exerts effects by increasing the local temperature within tumors - Does not affect surrounding healthy tissue and cells. - Minimal toxicity - Improves the results of combined therapy |
- Thermal stability is a highly critical parameter for photothermal materials - The morphology of a nanomaterial has significant effects on its physical chemistry and biological properties |
| Immunotherapy | - Helps in the induction of systemic immune response. - Includes both humoral and cell-mediated immunity. - Helps in tumor regression. - Induces tumor cell death without affecting normal healthy cells. - Targeting approach. - Better tolerance, less cytotoxicity and induction of tumor cell apoptosis. - Various types can be applied when combined together |
- Development of some side effects with cytokines in the body, such as diarrhoea, fatigue, pancytopenia, and tiredness. - Monotherapies are not capable of overcoming major events that can result in antitumor immunity in patients. - Some vaccines are costly, take a long time to induce an immune response, and are not reliable to use in fast-growing tumors. |
| Ultrasound | - Non-invasive and painless transmission of energy into the body - Helps to increase drug penetration - Leads to rapid termination of drug delivery - Increases therapeutic effect - Reduces adverse effects (less systemic absorption) - Very helpful in gene therapy - Greater patient satisfaction - Intact skin, no puncture, hence low risk of introducing infection - Reduces the interaction of the anti-neoplastic agent with healthy tissues |
- Can be time-consuming to deliver drugs - Stratum corneum must be intact for effective drug penetration - Strongly attenuated by bone - Minor tingling and irritation - Therapeutic agent variables, like surface charge, molecular weight, concentration, etc., may harm even the healthy tissues. - Ultrasonic rays may lead to DNS damage in cancer cell lines. |
3. INTRATUMORAL THERAPY
Conventional systemic chemotherapy is normally restrained for metastatic and recurrent tumors, which deliver the drug to the tumor site passively. This normally causes drug expulsion due to which cancer cell develops resistance toward the drugs, inevitably reducing therapeutic effects. Hence, local drug delivery directly to the tumor site may overcome these limitations. Recent literature depicts that local intratumoral therapy for oral cancer is becoming more common in practice to enhance patients' quality of life. Localized drug delivery to the tumor site is done by several methods, such as injecting the drug directly into the tumor, infusion through the catheter, vascular infusion, and intra-cavitary placement. Normally most of the tumor sites are injectable, but some of those may need specific technologies to complete the action. Intratumoral therapy can also be used for immunotherapy with cytokines, immune modulators, genes, and interferons [24-26]. This technique has many advantages and disadvantages, which are summarized in Table 2. Bakker et al., in their retrospective study, presented an analysis of three patients with already re-irradiated recurrent HNSCC, who were treated with intra-tumoral injections of holmium-166 microspheres(166HoMS) under ultrasound guidance with a dose of 70–100 Gy. They concluded that the palliation of HNSCC patients was minimally invasive and relatively safe [27]. Li J et al. studied the intratumoral application of a biodegradable hydrogel-controlled drug release system and observed the enhanced therapeutic effects and diminished side effects of suberoylanilide hydroxamic acid - cisplatin (SAHA/DDP) in the treatment of OSCC [28]. Essawy et al. experimented with 32 Syrian male hamsters after cancer induction and divided them into 4 groups; saline-intraperitoneal (IP), saline-intratumoral, doxycycline intraperitoneal (DOX-IP), and DOX-intratumoral, and evaluated them clinically, histopathologically, and immunohistochemically. DOX-1p and DOX-Intratumoral reported a significant decrease in the mean tumor volume, and intratumoral therapy was concluded as a suitable alternative to systemic administration [29].
4. PHOTODYNAMIC THERAPY (PDT)
PDT is one of the non-invasive types of phototherapy, which is characterized by its efficient therapeutic results. PDT is an established and approved aid to treat various malignant and non-malignant disorders. The most important advantage of PDT is that it reduces long-term morbidity [30]. PDT is a promising new method and has been shown to have significant parameters of success for treating oral cancer and dysplasia [31]. It mainly uses three components to attain the required effect on tumor cells, which are photosensitive compounds, photosensitizer light having a significant wavelength, and oxygen dissolved in the cells. PS are substances that can absorb light with a specific wavelength, leading to some photochemical or photophysical reactions. The components provide desirable effects within damaged tissues when used together, and form a highly reactive product, which causes remarkable toxicity eliciting cell death via apoptosis or necrosis, but ineffective when exposed to tumor cells individually.
PDT works on three main mechanisms [32]:
1. The direct cytotoxic damage to tumor cells,
2. Destruction of tumor vascularity, and
3. An inflammatory reaction that leads to a systemic immune response.
A PS must be a single material with a deep absorption in the red to the near-infrared wavelength (between 650 and 800 nm). PS are divided into porphyrins (5-aminolaevulinic acid (ALA) - induced protoporphyrin, chlorins (m-tetrahydroxyphenylchlorin - mTHPC, benzoporphyrin derivative, pyrophaeophorbide derivative HPPH, and tin (II) etiopurpurin, bacteriochlorin, phthalocyanines, synthetic and natural dyes [33]. Various clinical trials concerning 5-ALA, m-THPC, and porfimer sodium are being conducted for the treatment of cancer of the oral cavity [34]. In 2013, Rigual N et al. assessed the safety of 3-(1′-hexyloxyethyl) pyropheophorbide-a (HPPH) PDT in 45 patients with early HNSCC, and concluded it as a safe treatment [35]. Biel M.A. reported 276 patients with early head and neck carcinoma from 1990 till 2006, who were treated with PDT and were completely free of the disease after one PDT session [36]. Schweitzer and Somers evaluated the efficacy of dihematoporphyrin ether-mediated PDT in 30 patients with OSCC and observed complete remission in 80% of patients, and out of 24 cases, 11 were free of cancer at 2 years following PDT [37]. Vohra et al. studied the effectiveness of PDT on premalignant oral lesions and concluded this treatment as successful in the overall management of precancerous oral lesions [38]. Recently, Khan et al. investigated the capability of a simple smartphone-based device for imaging 5 (ALA)-induced protoporphyrin IX (PpIX) fluorescence for treating 29 patients with OSCC and found this approach to be effective [39]. Mimikos et al. reviewed various studies on the use of PDT in early oral cancer and demonstrated two cases to validate its effectiveness and stated that the treatment is effective, simple, easy, able to be be used again, and to result in minimal tissue scarring [40]. It has been found that this method, when combined with other techniques, resulted in improved results with minimal side effects. Due to all these properties, PDT is becoming more successful in the current therapy of oral cancer. The technique has many advantages and a few limitations, as summarized in Table 2.
5. PHOTOTHERMAL THERAPY (PTT)
Photothermal therapy (PTT) is a revolutionary technique that utilizes and encourages therapeutic near-infrared (NIR) laser photo-absorbers that generate heat for cancer cell thermal ablation. PTT is a local treatment modality that mainly employs the assistance of optical absorbing photothermal agents called photosensitisers, which absorb light energy, get excited, and convert it into heat energy that kills the targeted tumor cells. The agents get stimulated by electromagnetic radiations, like radiofrequency, visible light, microwave, or near-infrared irradiation. The technique is minimally invasive, resulting in minimal cytotoxicity. It has many advantages as compared to conventional drug delivery with few of its limitations (Table 2). It is independent of oxygen for the cellular and tissue interaction and uses light of a longer wavelength which does not harm normal cells and tissues. An ideal photosensitizer must have the least toxicity potential in the absence of light, cytotoxicity in the presence of the light of a certain wavelength, strong dispersibility in aqueous solutions, adequate and high photostability, high cross-section absorption, easy activity, and solubility in biocompatible solutions [41]. At present, several materials have been used for PTT, such as noble metal nanostructures, nano-carbons, transition metal sulphides/oxides, and organic nanoagents [42, 43]. Gold nanoparticles have been used as an innovative tool for diagnosis and drug delivery for different cancers due to their remarkable properties, which include bacteriostatic, anticorrosive, anti-oxidative, high stability, non-toxicity, non-immunogenicity, high permeability and retention effect, and high surface to volume ratio, which provide additional benefits. Inorganic nanoparticles are based on noble metals, like gold, which are potential photothermal agents with high therapeutic efficacy, non-toxicity, non-immunogenicity, high permeability, and high retention effect, helping in easy penetration and drug accumulation at the tumor sites [44, 45]. Sometimes PTT and PDT can also be combined to improve the therapeutic efficacy in oral cancer management [46]. Zou L et al. studied the efficacy of PTT with nanoparticles in the treatment of cancer and depicted that this therapy provided efficient results [47].
6. IMMUNOTHERAPY
Immunotherapy has been developed as a progressive innovation for the treatment of diverse types of cancer, including the locales of the head and neck. Immunotherapy counteracts the suppressing signaling pathways of the immune system and strengthens it by stimulating its specific components [23]. Immunotherapy is used as a remarkable approach to cancer therapy because it includes both humoral and cell-mediated immunity with a variable form of antigen receptors that can distinguish between normal and cancerous cells [48]. Immunotherapy can be classified into various types:
6.1. Active and Passive Immunotherapy
Active immunotherapy requires tumor cell attack, which views the tumor as a target. The cells used here are dendritic, NK, and cytotoxic T cells mainly. Whereas in passive immunotherapy, cell surface receptors are targeted, leading to the enhancement of the immune system and further to the development of antibody-dependent cell-mediated cytotoxicity (ADCC) [23].
6.2. Systemic Cell-mediated Immunotherapy
This is a non-specific type of immunotherapy that leads to the replacement of the entire immune system by a local/systemic antitumor response. It includes the following subtypes:
6.2.1. Adoptive cell transfer
In this, the T-cells of a patient are modified outside and then infused again. These T-cells are capable of detecting unique tumor antigens that contribute to cancer cell death.
6.2.2. Transfected dendritic cell therapy
In this, autologous dendritic cells are shredded along with the DNA of the patient’s tumor and reintroduced. Studies have shown that this approach leads to the induction of antigen-presenting cells without harming dendritic cells in the therapy of various HNSCC [48].
6.2.3. Cytokines immunotherapy
Cytokines are the special chemical mediators that direct the immune cells to interact with each other, resulting in a coordinated tumor cell response. This immunotherapy activates the immune cells of the patient through a complex pathway. Various cytokines have been used for the management of HNSCC for many years, e.g., IL-12, IL-2, GM-CSF, IRX-2, IFN-γ, etc [48, 49]. One of the limitations of these cytokines is the development of some side effects in the body, such as diarrhea, fatigue, pancytopenia, and tiredness [23].
6.3. Targeted Immunotherapy
Studies are being conducted to investigate the use of several tumor-associated antigens, which can be used as therapeutic targets in HNSCC. These can be either tumor-specific antigens, tumor-specific mutated proteins, or antigens overexpressed in tumor cells.
6.3.1. Monoclonal Targeted Antibody
Monoclonal antibodies are composed of components of human or murine antibodies linked to tumor-associated antigens exerting ADCC. Advantages of these therapeutic agents as compared to conventional drugs are their specific targeted action, better tolerance, less cytotoxicity, and induction of tumor cell apoptosis [50]. Vermorken et al. demonstrated that monoclonal antibodies, such as cetuximab and panitumumab, have been used as EGFR targeted therapies leading to the deregulation of EGFR, anti-apoptotic, invasive, metastatic, and angiogenic potential [51]. Antibodies have also been developed against MUC-1 and p53 in the treatment of various HNSCC and have shown effective results [52].
6.3.2. Checkpoint inhibitors
Certain inhibitory pathways suppress the activity of T-cells leading to the growth of the tumor. These are known as checkpoints. Blocking these checkpoints has been proven to prevent further tumor progression in various cancers, including oral cancer. Anti-CLTA-4 and anti-PD-1 antibodies are the most common checkpoint inhibitors used in this area. Others include mucin domain 3 (TIM-3), T-cell immunoglobulin, and lymphocyte-activation gene 3 (LAG3) [53]. Studies have shown that radiation therapy, along with PD-1 blockade, has a synergistic effect on cancer therapy [54]. A drug like Nivolumab has been shown to provide effective results when used as a checkpoint inhibitor in the treatment of recurrent HNSCC [55]. Pembrolizumab has been approved as a checkpoint inhibitor by the FDA to treat patients with recurrent HNSCC [56].
6.3.3. Vaccines
Vaccines are therapeutic measures that induce an antitumor immune response by acting as a tumor-associated antigen (TAA) and an immune-stimulatory adjuvant, resulting in immune sensitization to tumor antigens [57]. Vaccines direct T-cells to recognize and eliminate tumor cells. They contain variable antigens, such as DNA, RNA, peptides, dendritic cells, or whole cells [58]. The different types of vaccines are as follows:
- Antigen vaccines: They are made of unique patient tumor antigens that destroy cancer cells further.
- Dendritic cell vaccines: They are made up of dendritic cells that are capable of recognizing and attacking cancer cells.
- DNA or RNA vaccines: They have either DNA or RNA and help in tumor regression with effective results.
- Whole-cell vaccines: Rather than containing any specific antigens, these vaccines are developed from entire tumor cells.
Several vaccines are currently under review for HNSCC, like the DNA vaccine INO-3112, and the peptide vaccines Mucin-1 and Allo-Vax. Vaccines induce long-lived immunity with less toxic effects and can also be used in combination with other immunotherapies [59]. The only limitation is their high cost, long time taken to bring about the immune response, and inability to apply in the therapy of fast-growing tumors.
Immunotherapy is being used as a promising technology in the therapy of oral cancer nowadays. Like any other method, it also has its advantages and disadvantages, as described in Table 2.
7. ULTRASOUND IN ORAL CANCER TREATMENT
Ultrasound (US) is widely used in diagnostics and has many therapeutic applications. The ultrasound energy can be used non-destructively for effective therapeutic outcomes of drugs and genes for better cancer treatment. This drug delivery system works in a manner by which an acoustic energy deposition of ultrasound is used to create changes in vascular and cellular permeability to intensify the transport of drugs, as well as for positioning and activating drugs and genes via specially tailored vehicles and formulations. The acoustic cavitation acts as a triggering factor that leads to drug release at the targeting region. Multiple ultrasound drug-responsive systems include microbubbles (1-10 µm), nanobubbles, nanodroplets (< 1 µm), polymeric micelles, liposomes, and microemulsions [60, 61]. Research in the last decade has shown that a novel ultrasound-directed gene and drug delivery system has immense potential clinical therapeutic value for cancer patients in the future. The various mechanisms involved in sonoporation are [62]
(a) Production of hyperthermia - Ultrasound increases the medium’s temperature by absorption of the sound waves. With the increase in ultrasound frequency, the coefficient of absorption also increases, which leads to hyperthermia.
(b) Production of cavitation activities - In the medium where ultrasound is exposed, shallow space is created, which is termed as cavitation, and is divided into two categories.
- Inertial - Bubble grows rapidly depending on the ultrasound frequency, which collapses after reaching a certain diameter.
- Stable - Stably oscillating bubbles are formed which collapse due to sono-chemical reactions in extreme conditions of temperature above 5000K and pressure of 300bar.
(c) Acoustic streaming effects: Physical forces of the ultrasonic sound waves exert pressure that leads to displacement of ions and micro molecules.
(d) Perturbation of the drug carrier.
(e) Cell permeabilization and capillary rupture – Stresses are inflicted upon cells and tissues as a result of cavitation events.
This approach combined with the anti-epidermal growth factor receptor antibody resulted in growth inhibition of Ca9-22 cells in vitro. Maeda H et al. used a low dose of bleomycin and proposed that this novel application could be used successfully to treat oral cancer [63]. Curcumin exhibits anticancer and antioxidation properties at low doses, but at high dosages and given locally, it may be harmful to cancer cells. Lin HY et al., in their study, found that curcumin lipid micro-emulsions, when used along with low-frequency ultrasound, enhanced the effects of therapeutic results in oral cancer [64]. The microscopic imaging of the cells showed damaged and ruptured cells after treatment with the curcumin micro-emulsions (40–50 nm, containing a concentration of curcumin as high as 15 M), and the use of ultrasonic greatly amplified such effects, particularly on OSCC-25 cells. Yamatomo et al. demonstrated that boron neutron capture therapy along with low-intensity ultrasound in the treatment of OSCC resulted in an enhancement of the therapeutic efficiency of the drug [65]. Fan et al. reported the potential of low-intensity ultrasound (LIUS) and low effective dosages of doxorubicin (DOX) to inhibit the formation of this tumor by inhibiting cell proliferation, migration, and invasion [66]. US method has got several benefits as compared to conventional drug therapy but also has a few limitations as well (Table 2).
7.1. Trends in Tissue Healing and Repair
It is generally known that tumors are caused by complicated biological mechanisms, and that the tumor microenvironment (TME) plays a key role in regulating tumor biological behaviour. In the tumor microenvironment, cancer-associated fibroblasts (CAFs) are a group of activated fibroblasts with remarkable variability and plasticity [67]. CAFs are activated by diverse mechanisms, like FGF (fibroblast growth factor), PDGF (Platelet-derived growth factor), ROS (Reactive Oxygen species), RTK (Receptor tyrosine kinase), TGFβ (Transforming growth factor- β), and TNF (Tumor necrosis factor) [68]. Foster et al. elaborated evolving relationship between wound healing and tumor stroma. They concluded that cancer proliferation and metastasis could not occur if a tumor cannot grow without building a microenvironment [69]. Another important naturally occurring nutrient, betaine δ-valerobetaine (δVB), has antioxidant, anti-inflammatory, and anticancer properties. D’Onofrio et al. studied the possible synergism between δVB and butyrobetaine (γBB), and found encouraging results for the SIRT1-mediated apoptosis in Cal 27 cell lines [70].
7.2. Other Compounds for Targeted Treatment of Oral Cancer
During our journey to write a systematic review on current trends in the treatment of oral cancer, we came across various compounds that are being studied for targeted oral cancer therapeutics. Bundela et al. investigated various compounds, like vorinostat, andrographolide, nimbolide, resveratrol, deguelin, bortezomib, pterostilbene, colchicine, lovastatin, and berberine, as promising candidates for oral cancer treatment due to the relative importance of their targeted protein(s) in oral carcinogenesis [71]. In 2009, Fujita and colleagues investigated the role of the P53 gene in the effect of BNCT on oral cancer, concluding that it suppresses oral SCC cells in both p53-dependent and p53-independent ways [72]. Due to their outstanding biocompatibility and unique properties, fullerenes have shown enormous potential as photosensitizers in the photodynamic treatment of oral cancer. Lv Yanhong et al. examined the effects of exo-protoporphyrin-based sonodynamic treatment (PpIX-SDT) on oral squamous cell carcinoma cells in vitro and in vivo in 2017 [73]. Through cell cycle arrest and induction of cell death, PpIX-based SDT may effectively restrict the growth and proliferation of tongue cancer SAS cells.
8. LIMITATIONS
The current novel approaches mentioned above have been found to be useful and beneficial, but they also come with certain obstacles that still exist in the treatment of cancer. Photodynamic therapy can only be used for the treatment of cancer beneath the skin as the light used can penetrate up to 1/3rd of an inch or 1 cm [74]. Photothermal therapy causes damage to normal tissues, and at times, there is an insufficient therapy effect due to limited penetration of light [75]. In cancer immunotherapy, the limitations include unpredictable efficacy, difficulty in identifying significant biomarkers, development of resistance to the drug, and its expensiveness [76, 77]. During hyperthermia and thermoablation, the adequacy and homogeneity of nanoparticle accumulation at the cancer site is a critical issue, and also the nanoparticles do not penetrate consistently in poorly vascularized tissues [78]. To fully exploit this possibility, however, a better understanding of the paradoxical role of fibroblasts in malignancies is required. In most forms of solid tumors, cancer-associated fibroblasts (CAFs) are major micro-environmental components that are emerging as key participants in immune modulation that changes the tumor microenvironment. To fully exploit this possibility, however, a better understanding of the paradoxical role of fibroblasts in malignancies is required [69, 79].
8.1. Clinical Trials Undergoing/Completed on Various Targeted Drug Delivery Systems
Various clinical trials have been completed and/or going on various targeted drug delivery systems described above to investigate their exact role in the therapeutic aids of oral cancer (Table 3). Many drugs have been proven to show effective results in the field of oral cancer. However, large scale studies are needed to depict their efficacy and outcomes for future perspectives in oral oncology.
| S.No. |
NCT Number/ EudraCT Number |
Title of the Study | Status | Disease | Intervention | Phase | Sponsor, Collaborator and Location | Reference | ||
| Fast Dissolving Oral Thin Films | ||||||||||
| 1. | NCT03939364 | A Phase 1, Randomized, Double-blind, Placebo Controlled, Dose Escalation Study to Evaluate the Safety and Pharmacokinetics of SBS-101 when Applied Intraorally in Patients with Oral Premalignant Lesions (first most study) |
Yet to start | Oral pre-malignant lesions | Drug: SBS-101 (Isotretinoin Oral-Adhesive Film) Drug: Placebo |
Phase 1 | University of Alabama at Birmingham (UAB) United States Skyline Biosciences |
[99] | ||
| Hyperthermia and Thermoablation | ||||||||||
| 2. | NCT02567383 | Combination of Hyperthermia and Concurrent Chemoradiotherapy (CCRT) for Recurrent Head and Neck Cancer |
Recruiting | Head and Neck Cancer with high recurrency rate | Radiation: Radiation Device: Hyperthermia; Thermotron RF-8 Drug: Cisplatin Drug: Taxotere |
Phase 2 | Shin Kong Wu Ho-Su Memorial Hospital Taipei, Taiwan |
[100] | ||
| 3. | NCT03238378 | Salvage Brachytherapy with Interstitial Hyperthermia for Locally Recurrent Head & Neck Carcinoma Following Previously External Beam Radiation Therapy: A Prospective Phase II Study | Recruiting | Locally Recurrent Head and Neck Cancer | Radiation: Brachytherapy Other: Hyperthermia |
Phase 2 | Dept. of Radiation Therapy, University Hospital Erlangen Erlangen, Germany |
[101] | ||
| 4. | NCT03547388 | Moderate Whole Body Hyperthermia for Patients Undergoing Re-irradiation for Head and Neck Cancer - Influence on the Tumor Microenvironment | Completed | Head and Neck Neoplasms Recurrence Tumor |
Device: Moderate whole-body hyperthermia using water-filtered IR-A-radiation | Phase 1 | Klinik für Radioonkologie und Strahlentherapie Berlin, Germany |
[102] | ||
| Intratumoral Therapy | ||||||||||
| 5. |
EudraCT Number 2005-005170-80 |
A Randomized, Multicentre Therapeutic Confirmatory Study to Evaluate the Efficacy and Safety of Proxinium™ plus Best Supportive Care versus Best Supportive Care Alone in Patients with Advanced Squamous Cell Carcinoma of the Head and Neck who have Received at least One Anti-cancer Treatment Regimen for Advanced Disease | Ongoing France – ANSM Spain - AEMPS Completed Slovakia - SIDC (Slovak) Italy - Italian Medicines Agency |
Advanced Ep-CAM Positive Squamous Cell Carcinoma of the Head and Neck | Drug: Proxinium (intratumoral) |
Phase 3 | Viventia Biotech Inc. Canada France – ANSM Spain - AEMPS Slovakia - SIDC (Slovak)C Italy - Italian Medicines Agency |
[103] | ||
| 6. |
EudraCT Number 2019-003060-42 |
A Phase 2 Study in First Line Metastatic or Unresectable, Recurrent Head and Neck Squamous Cell Carcinoma to Evaluate Intratumoral MK-1454 in Combination with IV Pembrolizumab vs. IV Pembrolizumab Monotherapy |
Ongoing | Metastatic or Unresectable, recurrent HNSCC | MK-1454 (intratumoral) and Pembrolizumab – MK-3475 (i.v) Vs Pembrolizumab – MK-3475 (i.v) |
Phase 2 | Merck Sharp & Dohme Corp., a subsidiary of Merck &Co.,Inc Norway - NOMA UK – MHRA France - ANSM Spain - AEMPS |
[104] | ||
| 7. | NCT00634595 | A Randomized Phase II Clinical Trial of an Adenovirus-mediated Endostatin Gene (E10A) Combined with Cisplatin and Paclitaxel in Patients with Head and Neck Cancer |
Recruiting | Head and Neck Squamous Carcinoma Nasopharyngeal Carcinoma |
Drug: E10A E10A 1*10 (12) VP, intratumoral injection, d1 d8, Drug: Cisplatin 25 mg/m2/d ivd d3 d4 d5, Drug: Paclitaxel 160 mg/m2 ivd d3 (try again every 3 weeks for 4 cycles) |
Phase 2 | Cancer centre Sun Yat-sen University Doublle Bioproduct Inc |
[105] | ||
| Photodynamic Therapy | ||||||||||
| 8. |
NCT03638622 |
Image-guided Photodynamic Therapy (PDT) of Oral Cancer | Completed | Oral Cancer | Photodynamic Therapy | Phase 1 Phase 2 |
Sponsored by Massachusetts General Hospital in Collaboration with Jawaharlal Nehru Medical College National Cancer Institute (NCI) |
[106] | ||
| 9. | NCT00155337 | Photodynamic Therapy for Oral Leukoplakia and Erythro-leukoplakia | Completed | Oral Leukoplakia | photodynamic therapy | Phase 4 | National Taiwan University Hospital, Taipei, Taiwan | [107] | ||
| 10. | NCT00978081 | Photodynamic Therapy in Treating Patients with Premalignant or Early-stage Head and Neck Tumors | Completed | Head and Neck Cancer Precancerous Condition |
Drug: aminolevulinic acid hydrochloride | Phase 1 | Abramson Cancer Center of the University of Pennsylvania, Philadelphia, Pennsylvania, United States | [108] | ||
| 11. | NCT03090412 | Photodynamic Therapy with HPPH Compared to Standard of Care Surgery in Treating Patients with Oral Cavity Cancer |
Active, not recruiting | Stage I OSCC Stage II OSCC |
Drug: HPPH – - Other: Laboratory Biomarker Analysis - Drug: Photodynamic Therapy - Other: Quality-of Life Assessment - Procedure: Therapeutic Conventional Surgery |
Phase 2 | Roswell Park Cancer Institute, Buffalo, New York, United States University of Rocherster, Rochester, New York, United States |
[109] | ||
| 12. | NCT00028405 | Photodynamic Therapy System for Patients with Refractory/Unresponsive Solid Tumors Completed |
Completed | Liver Metastasis Pelvic Cancer Head and Neck Cancer Sarcoma Rectal cancer Breast Cancer Colorectal Cancer Mouth Cancer |
Drug: LS 11 (Taporfin Sodium) Device: Lumaflex Light Delivery Catheter |
Phase 1 | Medical Centre - University of Arizona, Wayne State University, East Carolina State University, University of Pennsylvania Albert Einstein Medical Center, Philadelphia, and Virginia Mason Medical Center, Washington, United States | [110] | ||
| 13. | NCT00470496 | Photodynamic Therapy Using HPPH in Treating Patients Undergoing Surgery for Primary or Recurrent Head and Neck Cancer |
Completed | Stage 1, Stage 2 and Recurrent Squamous Cell Carcinoma of Hypopharynx, Larynx, Lip and oral cavity, Oropharynx Paranasal Sinus and Salivary Gland along with 30 + More Cancers of Head and Neck Region | Drug: HPPH Drug: Photodynamic therapy Procedure: Conventional surgery |
Phase 1 | Roswell Park Cancer Institute, Buffalo, New York, United States | [111] | ||
| 14. |
EudraCT Number 2013-003133-14 |
An Open-label Study to Investigate the Tolerability, Pharmacokinetics and Antitumor Effect Following Photodynamic Therapy (PDT) with Single-ascending Doses of LUZ11 in Patients with Advanced Head and Neck Cancer | Ongoing | Advanced head and neck cancer | Photosensitizer: Redaporfin Dose - 50 J/cm2 of laser light at 749±3 nm |
Phase 1 Phase 2 |
Blueclinical - Investigacao e Desenvolvimento em Saude, Lda Portugal |
[112] | ||
| Photothermal Therapy | ||||||||||
| 15. | NCT00848042 | A Pilot Study of AuroLase(tm) Therapy in Patients with Refractory and/or Recurrent Tumors of the Head and Neck | Completed | Head and Neck Cancer | Device: AuroLase Therapy Infusion of AuroShell particles followed by laser illumination for photothermal ablation of target lesions |
Not Applicable | Nanospectra Biosciences Inc. Cancer Treatment Centres of American Western Regional Medicine Centre, Arizona Bayor College of Medicine, Texas |
[113] | ||
| Immunotherapy | ||||||||||
| 16. |
EudraCT Number 2017-000086-74 |
A Pilot Study of Personalized Biomarker-based Treatment Strategy or Immunotherapy in Patients with Recurrent/metastatic Squamous Cell Carcinoma of the Head and Neck "UPSTREAM" | Ongoing | Non-curable Recurrent/metastatic Squamous Cell Carcinoma of the Head and Neck | Drug: Monalizumab (i.v) Palbociclib (Oral) Afatinib Methotrexate (s.c) Paclitaxel (i.v) Docetaxel (i.v) 5- Fluorouracil (i.v) 5-Fluorouracil (i.v) Bleomycine (i.v) (s.c) Gemcitabine (i.v) Mitomycin (i.v) Durvalumab (i.v) |
Phase 2 | European Organisation for Research and Treatment of Cancer Belgium |
[114] | ||
| 17. |
CTRI/2010/091/000170 / |
An Observational Study to Evaluate the Safety and Efficacy of Cetuximab in Combination with Platinum-based Chemotherapy in the First-line Therapy of Recurrent/ metastatic Squamous Cell Carcinoma of Head and Neck (SCCHN) |
Open to Recruitment | Recurrent/ Metastatic Squamous Cell Carcinoma of Head and Neck (SCCHN | Cetuximab in combination with Platinum-based Chemotherapy Initial dose - 400 mg per m2 body surface area (BSA) Initial infusion rate 120 minutes. Subsequent weekly dose - 250 mg/m2 each, subsequent doses 60 minutes |
Phase 4 | West Bengal, Maharashtra, Rajasthan, AndraPradesh India |
[115] | ||
| 18. |
CTRI/2011/05/001716 Or NCT01265849 |
Phase III, Open-label, Randomized, Multi-center Study of the Effects of Leukocyte Interleukin, Injection [Multikine] Plus Standard of Care (Surgery + Radiotherapy or Surgery + Concurrent Chemoradiotherapy) in Subjects with Advanced Primary Squamous Cell Carcinoma of the Oral Cavity / Soft Palate Versus Standard of Care Only |
Active, not recruiting | Advanced Primary Squamous Cell Carcinoma of the Oral Cavity / Soft Palate Versus Standard of Care Only | Biological: Inj Leukocyte Interleukin (Multikine) Dose: 400 IU (as IL-2), 5x/week for 3 weeks Drug: Cyclophosphamide Drug: Indomethacin Dietary Supplement: Zinc Procedure: Surgery Drug: Cisplatin Radiation: Radiotherapy |
Phase 3 | Various Cancer Hospitals of Kerala, TamilNadu, Maharashtra, Rajasthan, Uttar Pradesh, Madhya Pradesh Simmons Cancer Institute at Southern Illinois University Springfield, Illinois, United States And 98+ more places |
[115] | ||
| 19. | NCT02296684 | Immunotherapy with MK-3475 in Surgically Resectable Head and Neck Squamous Cell Carcinoma |
Active | Cancer of Head and Neck Resectable Head and Neck Carcinoma |
Biological: MK-3475 (Neoadjuvant) Procedure: Surgery Radiation: Intensity modulated radiation therapy Radiation: Image-guided radiation therapy Drug: Cisplatin Biological: MK-3475 (adjuvant) Procedure: Peripheral blood |
Phase 2 | Washington University School of Medicine Memorial Sloan Kettering Cancer Centre, NY Dana Faber Cancer Institute, Boston Merck Sharp & Dohme Corp. |
[116] | ||
| 20. | CTRI/2019/09/021256 | A Clinical Trial to Study the Safety and Efficacy of Biosimilar Cetuximab in Patients with Recurrent Locoregional or Metastatic Squamous Cell Carcinoma of the Head and Neck | Not Yet Recruiting | Malignant neoplasm of overlapping sites of the lip, oral cavity and pharynx | Intervention Biosimilar Cetuximab Dosage Form: Initial dose 400 mg per m2 IV infusion over a period of 120 mins, subsequently 250 mg per m2 per week(visit 2 to visit18) as an IV infusion over a period of 60 minute |
Phase 3 | Various cancer hospital in India Alkem Laboratories Ltd Enzene Biosciences Limited |
[117] | ||
| 21. |
EudraCT Number 2017-003226-33 |
First-line Treatment of Locally Advanced HNSCC with Double Checkpoint Blockade and Radiotherapy Dependent on Intratumoral CD8+ T cell Infiltration (CheckRad-CD8) | Ongoing | Locally advanced head and neck squamous cell carcinoma (HNSCC) | Drug : Radio-Immuno-Therapy with Durvalumab (i.v) and Tremelimumab (i.v) |
Phase 2 | University Hospital Erlangen Medical Faculty of the Friedrich-Alexander University Erlangen-Nürnberg, Germany |
[118] | ||
| 22. | NCT03022409 | A Clinical Trial to Investigate Biomarker Effects of Pre-Surgical Treatment With DNA Damage Repair (DDR) Agents in Patients with Head and Neck Squamous Cell Carcinoma (HNSCC) who are Planned to Undergo Surgery that is Likely to be Followed by Radiotherapy and/or Chemotherapy | Not Yet Recruiting | Head and Neck Squamous Cell Carcinoma | Drug: Ceralasertib 160 mg BID Drug: Olaparib 100 mg or 150 mg BID |
Phase 1 | AstraZeneca Research Site Pittsburgh, Pennsylvania, United States Research Site Toulouse, France Research Site Changhua City, Taiwan |
[119] | ||
| Ultrasound | ||||||||||
| 23. | NCT02353260 | Efficacy and Safety of Ultrasound Hyperthermia Combined with Chemotherapy on Oral and Maxillofacial-Head and Neck Cancer | Recruiting | Head and Neck Cancer | Device: Ultrasound Hyperthermia (treated with therapeutic Ultrasound device on the 1st,3rd,5th,7th, and 9th day of each 21 days cycle) Drug: Docetaxel, Cisplatin, Fluorouracil |
Phase 2 | Xuzhou Central Hospital Shanghai Jiao Tong University. |
[120] | ||
CONCLUSION
Cancer has long been understood to be a disease in which cells invade surrounding tissues and spread to distant locations. Oral cancer is an emerging public health problem worldwide. Various conventional therapies are used for treating oral cancer, but they entail variable degrees of side effects both on the body as well as the cellular microenvironment. With advanced technology, many other aids have been introduced in the field of oncology to treat oral cancer with minimal side effects. All techniques described in this paper have been proven to show effective results in the therapeutic interventions of oral cancer. Moreover, they can be used even in combination with conventional drug therapy to show beneficial outcomes. Also, the likelihood of future novel and exciting antistroma targeted therapies will increase as more investigators investigate the important cellular, molecular, and immunologic factors within the tumor stroma, as well as make use of the vast knowledge related to wound healing that has clear after-effects with regard to the microenvironment. More research on CAFs and their relationship with tumor microenvironment will help in better understanding the targeted cancer therapeutics. Several clinical trials are being conducted as well as have been completed in this aspect to investigate definite results of these therapies, yet robust research is needed for further confirmation.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
STANDARDS OF REPORTING
PRISMA guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s website along with the published article.


