All published articles of this journal are available on ScienceDirect.
Neurosensory Assessment of Infraorbital Nerve Injury Following Unilateral Zygomaticomaxillary Complex Fracture – A Prospective Study
Abstract
Background:
This study aimed to assess the difference in the recovery pattern of branches of infraorbital nerve paraesthesia after zygomaticomaxillary complex (ZMC) fracture in both surgically and non-surgically managed patients.
Materials & Methods:
A prospective, observational study involving 31 patients with unilateral ZMC fracture - 15 in the surgical group (Group A) and 16 in the non-surgical group (Group B) was evaluated. These patients were assessed at the time of injury, 3-months follow-up, and 6-months follow-up for the sensory function of the infraorbital nerve. The assessment of paraesthesia by cotton wisp test, light touch monofilament test, and the cold thermal test was subjected to intra-group and inter-group correlation by McNemar test and Fischer's exact test. Repeated Measures ANOVA with post-hoc Bonferroni test for intra-group correlation and independent sample t-test for inter-group correlation were used for two-point discrimination.
Results:
A statistically significant improvement was noted on both 3 and 6 months follow-up in the malar region in group A. Other statistically significant improvements were noted only on 6 months follow-up in the infraorbital region in group A. On the 2-point discrimination test, all the facial regions showed significant improvement in both the groups over 3 months and 6 months of follow-up.
Conclusion:
There was a significant improvement in the infraorbital nerve sensory function following ZMC fracture over 6 months; however, the surgical intervention showed no statistical significance. Further, it can also be concluded that the inferior palpebral branch of the infraorbital nerve shows maximum functional disruption resulting in a higher incidence of paraesthesia in the infraorbital and malar region.
1. INTRODUCTION
The zygoma, a principal structure of the lateral mid-face, is a thick, strong, and roughly quadrilateral bone that contributes significantly to the stability of the midfacial region. It forms one of the major buttresses of the facial skeleton. Its articulation with the frontal, sphenoid, and maxillary bones forms the lateral wall and floor of the orbital cavity.
Zygomaticomaxillary complex (ZMC) fractures are reported to be the most common fractures and second only to nasal bone fractures by prevalence reports [1]. Fracture of the zygoma typically presents as periorbital edema and ecchymosis, flattening of the malar prominence, and palpable step at the infraorbital or frontozygomatic region [2]. In the initial stage of non-displaced or minimally displaced fractures, some degree of neurosensory disturbance of the area supplied by the infraorbital nerve is often noticed. Thus, paresthesia over the infraorbital nerve distribution post-trauma has even been considered indicative of a fracture.
Infraorbital nerve injury is predominantly a compression type of injury in ZMC fracture due to the displacement of the infraorbital rim, resulting in nerve entrapment as it leaves the infraorbital canal and/or foramen (Fig. 1). The prevalence of infraorbital nerve paraesthesia secondary to ZMC fractures is reported to be present in 24% to 94% of the cases [3]. Tissue swelling or edema, laceration of tissues, and nerve compression or traction due to fracture fragments can also lead to symptoms that may vary from hypoesthesia, dysesthesia, hyperesthesia, and paraesthesia to numbness of the affected site; i.e., nose, upper lip, and loss of sensation of teeth.
Various studies have reported long-term neurosensory discrepancies of various branches of infraorbital nerve post-trauma [4, 5]. Persistent disturbance in the area innervated by the infraorbital nerve, which is mostly a neuropraxia type of nerve injury, can result from delayed or inadequate management of these fractures. Some studies have shown that long-term disturbances in infraorbital nerve function were present in nearly 50% of their cases, while others have observed a much lower rate of 10% at one-year follow-up [6, 7].
Depending on the functional restriction and esthetic deformity, the treatment for ZMC fracture ranges from conservative management to open reduction and internal fixation (ORIF). Most of the surgeons agree that conservative treatment should be reserved for fractures with minimal displacement, in which cases the patient is managed by instructing them to be on soft diet for a period of 4 to 6 weeks [8]. Meanwhile, surgical treatment in the form of ORIF has been found to be an effective treatment modality for displaced ZMC fracture with functional and/or esthetic deformity [9]. Various extra-oral and intra-oral approaches have been described in the literature for reduction of the displaced ZMC fracture. Extra-oral approaches like Gillies temporal approach or Keen’s approach are often used for severely displaced fractures [10, 11]. Whereas, numerous studies have shown satisfactory treatment ooutcomes with intra-oral approaches in relatively lesser displaced fractures [12-14]. Similarly, with regard to fixation, one-point fixation at zygomaticomaxillary buttress has shown satisfactory outcome in minimally displaced ZMC fracture [15, 16]. Other fixation techniques including two-point fixation with plating at zygomaticomaxillary buttress and frontozygomatic suture, and three-point fixation with additional plating at infraorbital rim have also been extensively used and studied by various surgeons [17].
This study aims to clinically evaluate the sensory changes in areas of infraorbital nerve distribution following unilateral ZMC fractures and determine the recovery rate of paresthesia in surgically and conservatively managed cases by employing easily accessible and economical techniques of sensory assessment over a 6-months period.
2. MATERIALS AND METHODS
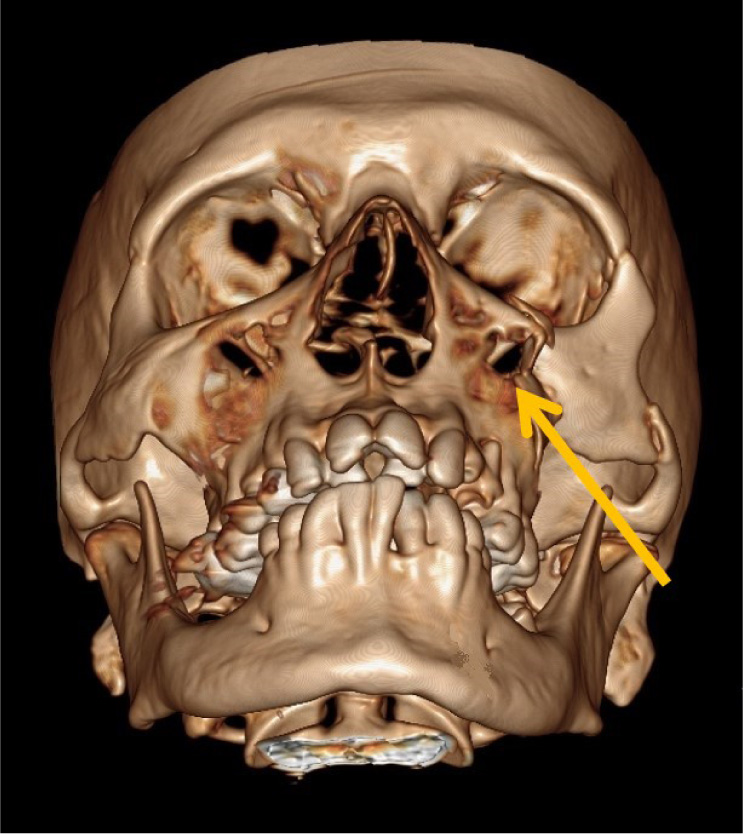
After obtaining Institutional Ethical Committee approval (KH-IEC: 838/2017), an observational, prospective study was conducted on patients presenting to our Tertiary care trauma centre. The patients presenting with clinically and radiographically diagnosed unilateral ZMC fractures from December 2017 to June 2019 were included in the study after their informed consent. The patients were evaluated for ZMC fracture clinically by the author S.G. or A.S. Computed tomography was performed with axial and coronal sections, and reconstructed images were obtained, which were evaluated by experienced radiologists. Inclusion criteria consisted of patients aged above 18 years of age reporting with unilateral ZMC fracture (Fig. 2). Patients with follow-up data of 6 months were only included in the study. Exclusion criteria consisted of patients with comminuted ZMC fractures or combined with other mid-face fractures or associated head injuries, bilateral ZMC fractures, fractures requiring orbital floor exploration and patients presenting with pre-trauma trigeminal or infraorbital nerve paraesthesia.
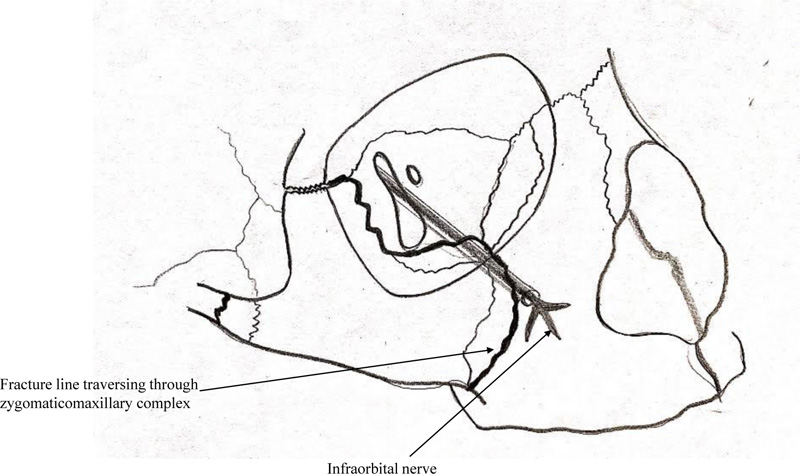
The indications for surgical management were the presence of one or more the following – restricted mouth opening or mandibular movements, cosmetic deformity, non-resolving paraesthesia after one week of trauma, gross mobility of fracture segments. Patients with unilateral ZMC fracture not having any of the above-mentioned indications for surgery, were managed conservatively. Additionally, any patient not consenting for surgical management was managed conservatively.
Sample size was calculated based on the values of electrical conduction at 6 months from previous study (effect size of 1.4). Sample size was estimated to be 12 per group with 90% power and 95% confidence interval. Considering a 10% attrition, the sample size was inflated to 15 per group.
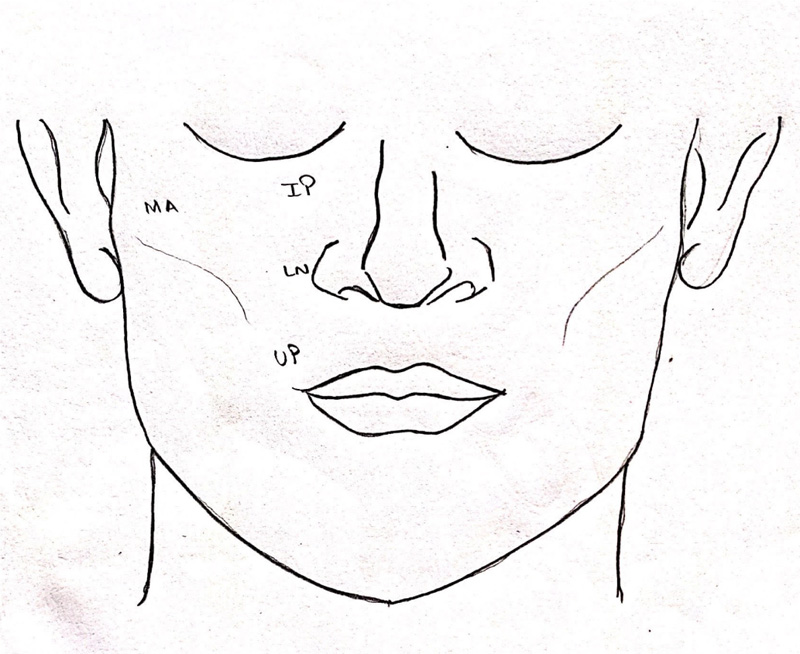
Demographic data were collected for all the patients, which included age, gender of the patient, mode, and cause of injury, time of injury, time elapsed between injury incurred, and initial management or treatment. After obtaining patient consent and familiarizing the patient with the armamentarium being used, four areas of the mid-face region supplied by the infraorbital nerve were tested bilaterally: infraorbital, lateral nasal, malar, and upper lip (Fig. 3). These areas were assessed using three tests – cotton wisp test, light touch monofilament test, and cold thermal test. The presence of sensation by all the three tests mentioned above was marked as “+,” and absence of sensation or sensory dysfunction by one or more of the tests was marked as “-,” which was indicative of paraesthesia in the area evaluated. Additionally, a two-point discrimination test was done, which was marked in millimeters. A blinded observer, R.L. did all the assessments.
2.1. Light Touch Monofilament Test
With the patient's eyes closed, a sterile 2-0 ethilon monofilament suture of prefixed length (10 mm) was placed perpendicular to the area and pressed till it just began to bend, and the patient was asked if they could feel the sensation.
2.2. Cotton Wisp Test
With the patient's eyes closed, a cotton wisp was stroked across the face from right to left and vice versa in all four regions. After each stroke, the patient was asked to indicate the direction verbally.
2.3. Cold Thermal Test
Diethyl ether was placed on a sterile cotton applicator tip. Once crystals formed onto it, it was applied to each region for not more than 3 seconds each to evaluate sensory function.
2.4. Two-point Discrimination
At the beginning of the test, both caliper points were held together (zero distance) onto the skin gently, and the patient was asked to identify if the contact is with 1 point or 2 points. The threshold distance was identified, increasing 1 mm of increment with each successive step till 2 points were felt separately. Further applications were made to overshoot this distance by 2 to 3 mm; then, the process was reversed from that point in 1-mm increments until the patient again no longer perceived two separate contacts.
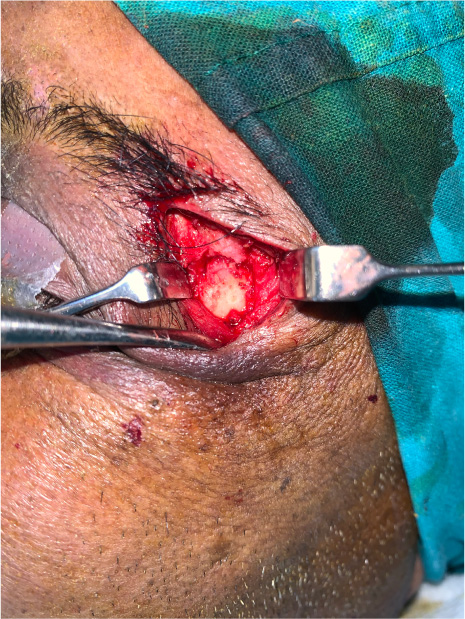
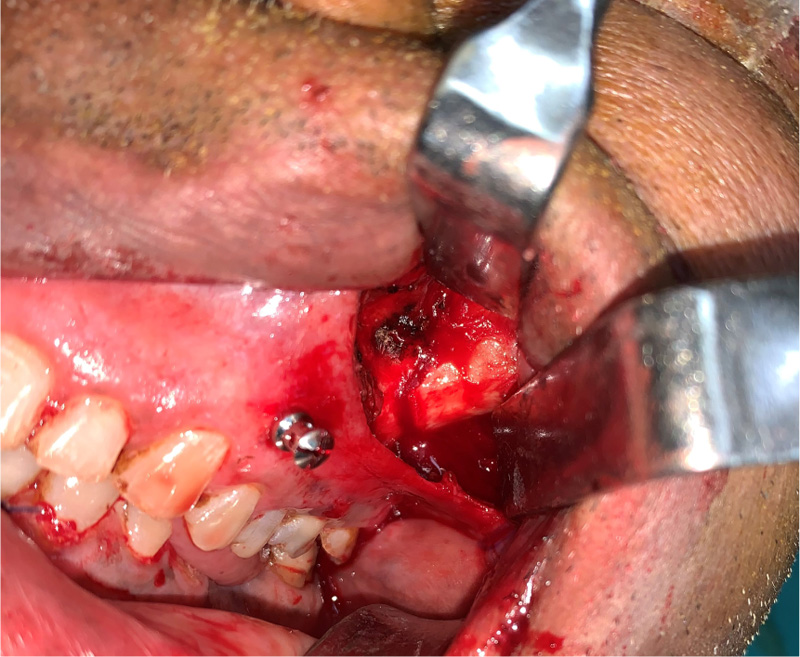
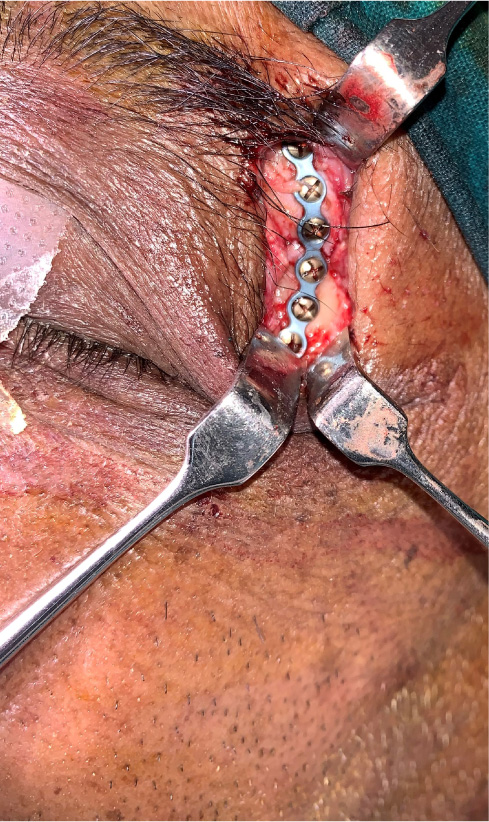
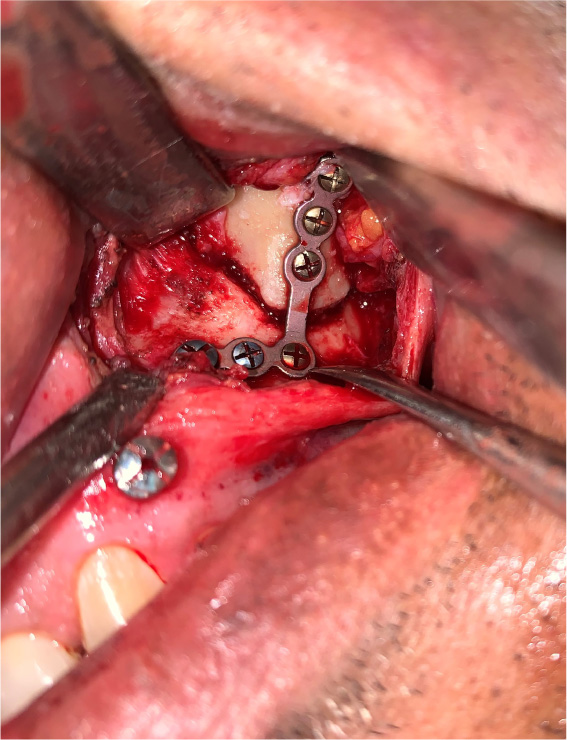
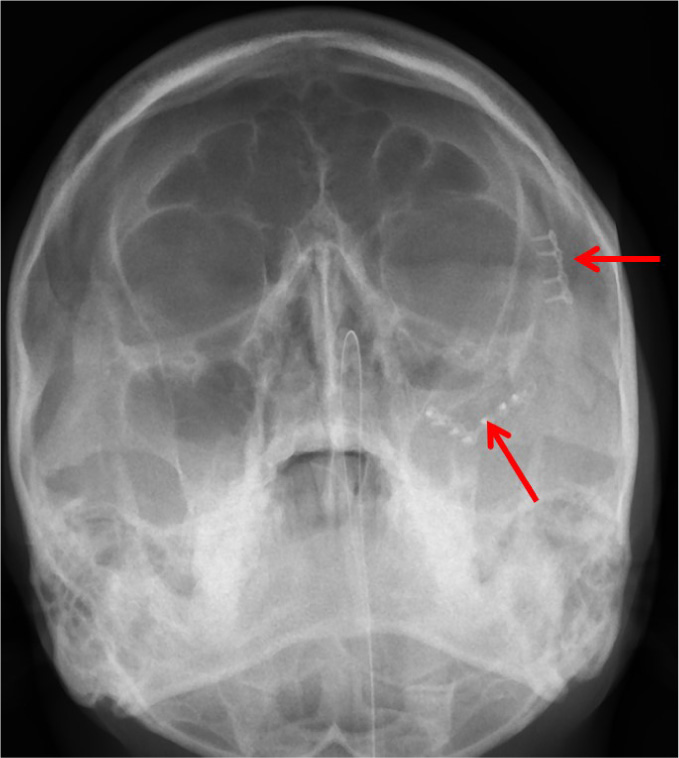
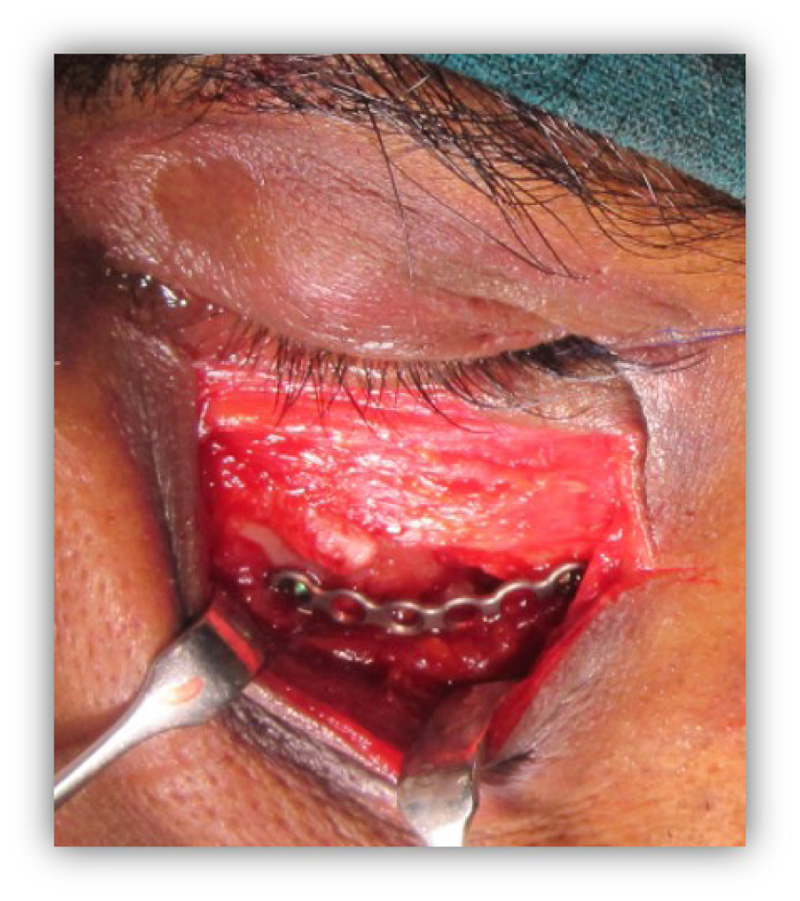
The patients in the surgical group (cases) were operated by the author S.G. or A.S. A standard lateral brow incision was used to access the frontozygomatic suture and upper buccal sulcus incision was used to access the zygomaticomaxillary buttress. (Fig. 4). After exposure and reduction of the fractures, sequential 2-point fixation was done by first plating at fronrozygomatic suture, followed by plating at zygomaticomaxillary buttress (Figs. 5-8). When indicated, an additional subciliary incision was used for accessing the infraorbital rim for 3-point fixation. The need for 3-point fixation was assessed by evaluating the reduction ater 2-point fixation and palpating for any residual step at infraorbital rim. (Figs. 9, 10) The patients being managed conservatively (controls) were advised to be on soft chew oral diet for a period of 6 to 8 weeks with a regular follow-up at 2 weeks for 1st 2 months, followed by monthly follow-up for 6 months period.

The patients were evaluated for paraesthesia on the day of trauma, at 3 months follow-up and 6 months follow-up in both the groups. The paraesthesia and recovery were assessed in the four anatomical areas supplied by the infraorbital nerve, i.e., infraorbital, lateral nasal, malar, and upper lip, in both groups. (Fig. 3) The final data were tabulated in MS Excel Sheet and analyzed utilizing software SPSS (Statistical Package for Social Sciences) Version 22.0. The assessment of paraesthesia by cotton wisp test, light touch mono filament test, and cold thermal test was subjected to intra-group and inter-group correlation by McNemar test and Fischer's exact test. For two-point discrimination repeated Measures ANOVA with post-hoc Bonferroni test for intra-group correlation and independent sample t-test for inter-group correlation was used. The study was conducted in line with Good Clinical Practice guidelines and in line with the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement [8] (Fig. 11).
3. RESULTS
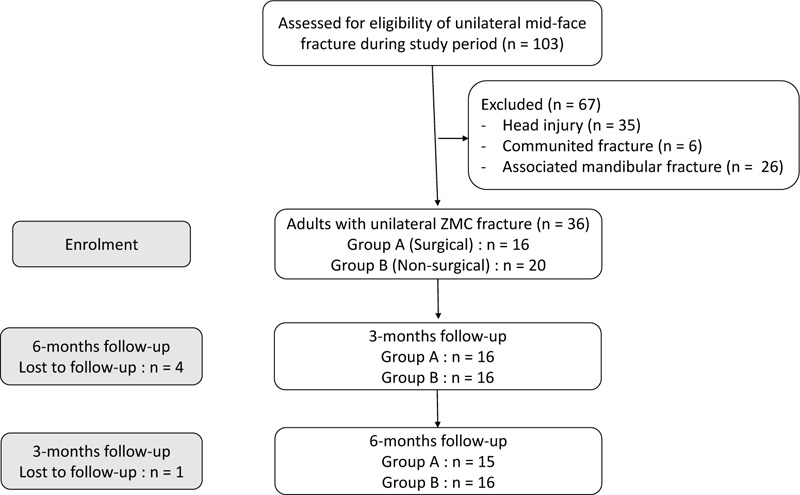
Thirty-six patients with displaced unilateral ZMC fracture during the study period were enrolled in the study. Five patients were lost to follow-up. Fifteen patients with ZMC fracture underwent open reduction and internal fixation (ORIF) as per the indications included in the surgical group (Group A), and the remaining 16 patients who were managed conservatively were included in non-surgical group (Group B). All the patients were followed up for six months.
Patients‘ age ranged from 20 to 76 years. In group A, 11 patients were male, while in group B, 14 were male. The majority of the patients were involved in motor vehicle road traffic accident (n=24), followed by fall from height (n=5) and inter-personal violence (n=2). An almost equal distribution was noted in the affected side, with 16 patients (51.6%) presenting with right-sided zygoma fractures and 15 (48.3%) with left-side zygoma fractures.
At the time of trauma, five (33.3%) patients reported normal sensation in group A, while 10 (62.5%) patients reported normal sensation in group B with regard to the infraorbital region. In relation to the malar region, only three (20%) patients reported having normal sensation in group A, whereas 7 (43.8%) patients had normal sensation in group B. There was a comparatively lesser disruption in sensation in relation to the lateral nasal and the upper lip region in both the groups. In relation to lateral nasal region, nine (60%) in group A and nine (56.2%) in group B had normal sensation. The upper lip region showed the least incidence of paraesthesia post-trauma as 14 (93.3%) patients in Group A, and 10 (62.5%) patients in Group B had normal sensation (Table 1).
| Absence of Paraesthesia in Different Facial Sub-Units |
Group A N (%) |
Group B N (%) |
P-value‡ |
|---|---|---|---|
| Infraorbital region (IP) | - | - | - |
| Baseline | 5(33.3) | 10(62.5) | 0.104 |
| 3-months follow-up | 9(60) | 12(75) | 0.458 |
| 6-months follow-up | 13(86.7) | 14(87.5) | >0.99 |
| P-value† (1 vs 2) | 0.125 | 0.727 | |
| P-value† (1 vs 3) | 0.008 | 0.125 | |
| Lateral nasal region (LN) | - | - | - |
| Baseline | 9(60) | 9(56.2) | 0.833 |
| 3-months follow-up | 13(86.7) | 13(81.2) | >0.99 |
| 6-months follow-up | 14(93.3) | 13(81.2) | 0.6 |
| P-value† (1 vs 2) | 0.125 | 0.125 | |
| P-value† (1 vs 3) | 0.63 | 0.219 | |
| Upper lip region (UP) | - | - | - |
| Baseline | 14(93.3) | 10(62.5) | 0.083 |
| 3-months follow-up | 15(100) | 11(68.8) | 0.043 |
| 6-months follow-up | 15(100) | 13(81.2) | 0.226 |
| P-value† (1 vs 2) | - | >0.99 | |
| P-value† (1 vs 3) | - | 0.25 | |
| Malar region (MA) | - | - | - |
| Baseline | 3(20) | 7(43.8) | 0.252 |
| 3-months follow-up | 11(73.3) | 9(56.2) | 0.32 |
| 6-months follow-up | 14(93.3) | 11(68.8) | 0.172 |
| P-value† (1 vs 2) | 0.008 | 0.5 | |
| P-value† (1 vs 3) | 0.001 | 0.125 |
Amongst all the observations, a statistically significant improvement was noted on both 3 and 6 months follow-up in the malar region in group A (p = 0.008 and p = 0.001, respectively). Only other statistically significant improvement was noted on 6 months follow-up in the infraorbital region in group A (p=0.008). No other statistically significant difference was seen Table 1.
For the 2-point discrimination test, the initial best measurement in Group A was noted in the upper lip region with a mean of 20.4mm, while the lateral nasal showed the best measurement in Group B with a mean of 18.5mm. All the four regions, i.e., infraorbital, lateral nasal, upper lip, and malar region, showed significant improvement in readings on 2-point discrimination test in both the groups over 3 months and 6 months follow-up. On post-hoc test, all the regions, except for upper lip region in Group A, showed significant improvement over 6 months follow-up compared to 3 months follow-up observation. Upper lip region in the surgical group showed significant improvement on 3 months follow-up, but there was no further significant improvement in 6 months follow-up.
Comparing group A with group B regarding the 2-point discrimination test, a significantly lower value was noted for the group B at the time of trauma and 6 months follow-up. This was in contrast to the findings noted by the other three sensory tests, which showed lesser disruption of sensation in group A. No other significant difference was seen in Table 2.
|
Group A Mean± SD |
Group B Mean± SD |
P-value‡ | |
|---|---|---|---|
| Infraorbital region (IO) | - | - | - |
| Baseline | 21.27±2.52 | 20.13±2 | 0.171 |
| 3-months follow-up | 19±2.24 | 19.56±1.93 | 0.459 |
| 6-months follow-up | 18.33±2.5 | 18.31±2.18 | 0.98 |
| P-value† | <0.001 | <0.001 | |
| Post-hoc test | 1>2>3 | 1>2>3 | |
| Lateral nasal region (LN) | - | - | - |
| Baseline | 20.8±2.24 | 18.5±1.86 | 0.004 |
| 3-months follow-up | 18.73±2.25 | 17.56±1.59 | 0.103 |
| 6-months follow-up | 17.93±1.75 | 16.56±1.26 | 0.018 |
| P-value† | <0.001 | <0.001 | |
| Post-hoc test | 1>2>3 | 1>2>3 | |
| Upper lip region (UP) | - | - | - |
| Baseline | 20.4±1.72 | 19.75±2.29 | 0.382 |
| 3-months follow-up | 18.33±1.72 | 18.44±2.22 | 0.885 |
| 6-months follow-up | 18±3 | 17.56±2.48 | 0.66 |
| P-value† | 0.008 | <0.001 | |
| Post-hoc test | 1>2,3 | 1>2>3 | |
| Malar region (MA) | - | - | - |
| Baseline | 20.67±2.09 | 19.38±1.89 | 0.082 |
| 3-months follow-up | 19.4±2.13 | 18.56±1.67 | 0.232 |
| 6-months follow-up | 18.07±2.09 | 17.88±1.86 | 0.789 |
| P-value† | <0.001 | <0.001 | |
| Post-hoc test | 1>2>3 | 1>2>3 |
Two patients in group A also had orbital floor fracture with muscle entrapment diagnosed clinically (FDT positive, persistent diplopia) and radiographically and, underwent orbital floor reconstruction. No complications like surgical site infection or hardware failure, or functional restriction were noted during the six-month follow-up.
4. DISCUSSION
Our study aimed to assess the improvement in the sensation of the areas supplied by the infraorbital nerve after a surgical intervention or conservative management in cases of ZMC fracture. Based on the results, more percentage of subjects showed improvement in sensory function after surgical intervention as compared to the subjects managed by conservative methods. This improvement was more significant in the infraorbital and the malar region, supplied by the inferior palpebral branch of the infraorbital nerve. Fractures of the zygoma are common, and the fracture line tends to pass through the weaker structures of the midface like infraorbital foramen. The nature of injury and the time elapsed between injury and treatment undertaken were found to directly impact the recovery of sensation [6, 19].
The infraorbital nerve is mainly subjected to compression type of injury or neuropraxia owing to impacted bony fragment or edema in the surrounding tissues. Different nerve fibers exhibit different susceptibility to compression and ischemia, two of the leading causes of traumatic nerve injury post ZMC fracture. The A-δ fibers (myelinated but smaller in diameter) and C fibers (unmyelinated) are responsible for nociception (pain, temperature). Being more primitive, the unmyelinated fibers are less predisposed to injury by compression than the large-diameter A-δ fibers (myelinated) responsible for mechanoception. Thus, following a compression type of injury, it is common to find intact pain and temperature discrimination sensation but impaired light/moving touch sensation.
Different studies have reported long-term sensory disturbance incidence to be from 24% to 94% [1, 6, 19-22]. These neurosensory disturbance has been reported in the form of paraesthesia or hyperesthesia or dysesthesia. In the study by Taicher et al. involving 183 patients with ZMC fracture, 80% of the patients reported having some sensory disturbance, with hyperesthesia being the most common disturbance (70%) [21]. Whereas dysesthesia was reported in up to 80% of patients with ZMC fracture in other studies [19, 20]. Findings in our study showed that 68% of patients in both the (21 out of 31) groups had some form of sensory disturbance.
One of the primary indications for surgical intervention in ZMC fracture is the persistent paraesthesia of infraorbital nerve. Any maneuver undertaken to relieve the mechanical impingement of the nerve by the displaced bone segments should result in the improvement of sensory nerve function. However, the surgical procedure results in inflammatory edema, which can cause compression of the infraorbital nerve, resulting in post-surgical paraesthesia. In earlier studies, the nerve's recovery rate was considered good; however, later studies have proved it to be controversial [19-21].
A 3 months minimal interval was chosen by Virens as most regenerative histologic reactions to trigeminal nerve injury are expected to recover by that time [23]. According to Donoff, it is unlikely for a nerve to recover if it has not recovered in six months spontaneously [24]. Hence in our study, we chose a follow-up interval of 3 months and 6 months duration to evaluate for improvement in sensory function and evaluate for residual paraesthesia.
The need for surgical management by fracture reduction and fixation was 51% in our study (16 out of 31). The need for fracture reduction in ZMC fractures has been found in other studies between 60% to 95% of cases [25-28]. Considering the difference in the sample size of the cases mentioned, a similarity can be constituted in the original study and those mentioned. In the study by Benoliel, a significant improvement in the infraorbital nerve neurosensory function was noticed in patients who underwent ORIF compared to the patients who underwent only reduction [25]. Routine use of mini plates for 3-dimensional stability of the fractured segment allowed for nerve decompression, facilitating repair and recovery [29, 30].
Contrary to this, Schultze-Mosgau et al., in their study, found that 55% of patients with mid-face fractures showed worsening of the sensory function of infraorbital nerve following osteosynthesis [31]. Similarly, Peltomma and Rihkanen concluded that in patients with minimal fracture displacement, the sensory dysfunction might be enhanced by surgical intervention.5 Interestingly, other studies also observed that the group not treated surgically had comparably similar recovery rates of infraorbital nerve paraesthesia, indicating spontaneous recovery as mentioned in previous studies [32].
The primary objective of our study was to compare the change in sensory function of infraorbital nerve in the surgical and non-surgical groups. Even though both the groups showed improvement in sensory function over 3-months and 6-months follow-up, there was no statistically significant difference noted between these groups. In addition, 2-point discrimination test was also done, which could assist in determining the functional disturbance of the patient in assessing the texture of food. Similarly, in this test, both the groups showed improvement in sensory function, but there was no significant difference in both the groups.
For all sensory modalities, the conservatively managed group presented with a similar recovery rate of the nerve function as the surgically managed group and only moderately less than the surgical group of patients. Since all the surgically managed patients were treated with miniplate fixation, the comparison in intragroup with reduction only or intraosseous wiring as done in other studies to determine the role of mode of treatment in recovery of the nerve was not possible. However, the similarity in the recovery rate of both the surgically managed and conservatively managed patients in this study was found to be similar to that noted by Benoliel and De Man et al. [25] It is postulated that this might be so because those managed conservatively are pre-selected population of patients with minimally displaced fractures exhibiting lower rates of functional deficits at the time of presentation.
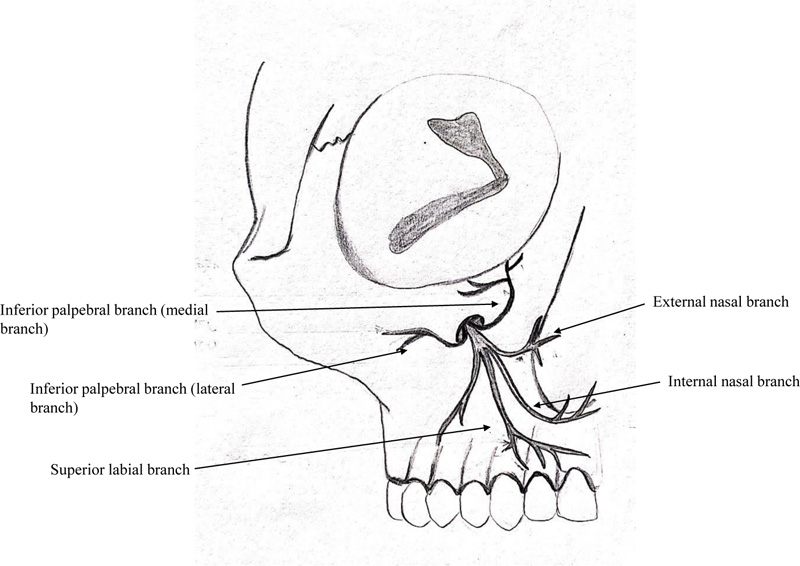
The infraorbital nerve produces four main branches, external nasal, internal nasal, inferior palpebral, and superior labial branches (Fig. 12). The inferior palpebral branch is generally bifurcated, giving off a medial and a lateral branch (58.1%) [33]. The secondary objective of this study was to determine whether there was any pattern to the disruption of various terminal branches of the infraorbital nerve in the ZMC fracture. Based on these branches, four different areas of mid-face regions were assessed separately, namely, malar region (lateral branch of inferior palpebral branch), infraorbital region (inferior palpebral branch), lateral nasal (external nasal branch), and upper lip (superior labial branch). In both groups combined, at the time of trauma, the maximum sensory disruption was noted in the malar region (68%) followed by the infraorbital region (52%). Subsequently, these two regions showed significant improvement by surgical intervention. On 2-point discrimination test, the infraorbital region in both the groups had the worst measurement at the time of trauma. However, all four facial sub-divisions showed significant improvement in 2-point discrimination test over a 6-months follow-up. Different areas of sensory abnormalities in the region of the infraorbital nerve indicate that different fascicles within the nerve can be affected differently, depending on the mechanism of injury and location of the compression [4]. The knowledge regarding the branch at the highest risk of nerve injury can affect the treatment plan, and measures can be undertaken to relieve the decompression in the affected branch. Based on our results, we can infer that the infraorbital branch is at the highest risk for sensory nerve damage and shows marked improvement in function with timely surgical intervention.
A drawback of this study is that no comparative group of the patient had any form of pharmaceutical therapy for sensory nerve dysfunction. Studies have been done evaluating the efficacy of various pharmaceutical agents like an antiepileptic drug to relieve paraesthesia [34]. So future studies incorporating an additional arm receiving pharmaceutical therapy can further help in enhancing the treatment outcomes for infraorbital nerve paraesthesia. Another drawback of the study is the limited study sample and the follow-up period. Further studies with bigger sample size and longer period are needed to determine further the effects of the infraorbital nerve's long-term neurosensory deficit, etiology, and methods to prevent the long-term deficit. In doing so, not only will we achieve a better understanding of nerve injury prevention and regeneration but also its clinical significance to the patient in their day-to-day life.
Through this study, the authors can conclude a significant improvement in the sensory function of infraorbital nerve following ZMC fracture over a 6-months period; however, the surgical intervention showed no statistical significance. Further, it can also be concluded that the inferior palpebral branch of infraorbital nerve shows maximum functional disruption resulting in a higher incidence of paraesthesia of infraorbital and malar region.
CONCLUSION
There was a significant improvement in the infraorbital nerve sensory function following ZMC fracture over 6 months; however, the surgical intervention showed no statistical significance. Further, it was also concluded that the inferior palpebral branch of infraorbital nerve shows maximum functional disruption resulting in a higher incidence of paraesthesia of infraorbital and malar region.
LIST OF ABBREVIATIONS
| ZMC | = Zygomaticomaxillary Complex |
| SPSS | = Statistical Package for Social Sciences |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Institutional Ethical Committee approval, Kasturba Hospital, Manipal. (KH-IEC: 838/2017).
HUMAN AND ANIMAL RIGHTS
No animals were used in the study that are the basis of this research. All the humans used were in accordance withthe ethical standards of the committee responsible for human with the Helsinki Declaration of 1975.
CONSENT FOR PUBLICATION
Informed consent has been obtained from the participants involved.
STANDARDS OF REPORTING
STROBE guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available in the Mendeley Repository at Pentapati, Kalyana; Singh, Anupam; gadichera, srikanth (2022), “Neurosensory Assessment of Infraorbital Nerve Injury Following Unilateral Zygomaticomaxillary Complex Fracture ”, Mendeley Data, V1, doi: 10.17632/j4hw68hty2.1.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest regarding the publication of this article.
ACKNOWLEDGEMENTS
Declared none.


