Surface Morphological Changes and Predisposition to Staining in Dental Enamel Bleached with Different Hydrogen Peroxide Concentrations
Abstract
Background:
The tooth bleaching treatment can cause structural changes in the surfaces of the teeth; these changes can increase the absorption of staining agents.
Purpose:
This study assessed surface morphological changes and predisposition to staining in dental enamel bleached with different hydrogen peroxide (HP) concentrations, with or without the use of a light source (LS).
Methods:
25 bovine incisor specimens were divided into five groups (n = 5): Control- no treatment; HP35 - hydrogen peroxide 35%; HP35+LED - hydrogen peroxide 35% + light emission; HP20 - hydrogen peroxide 20%; and HP7 - hydrogen peroxide 7,5%. Twenty days after bleaching, the specimens were immersed in staining solutions four hours a day for 28 days. The morphological alterations of the bovine enamel surface were evaluated by means of scanning electron microscopy, X-ray dispersive energy spectroscopy and predisposition to the staining of the brightened enamel by means of colorimetry.
Results:
ANOVA with Tukey's test (p<0.05) showed that HP7 had the highest ΔL values (p=0.176) (brightest), with a better lightening effect. The bleached groups exhibited morphological changes in the enamel. The groups did not exhibit significant changes in oxygen, calcium, and phosphorus values (p=0.020). The presence or absence of light was not significant (p=0.007) for the predisposition to staining in bleached teeth.
Conclusion:
The time of exposure to the staining solution was significant for staining bovine dental enamel. High concentrations of HP were not necessary for achieving effective bleaching. HP caused an increase in enamel porosity and depressions. The light source did not influence bleaching.
1. INTRODUCTION
In the last decade, there has been an increasing demand for cosmetic dentistry [1]. A recent systematic review observed a positive impact on smiling, laughing and showing teeth without embarrassment after bleaching treatment [2]. Thus, the color of the teeth contributes significantly to the appearance of a person, which is determined by the optical and chromatic properties of dentin and enamel [3]. Intrinsic and extrinsic factors in dentin or enamel, or both, can cause staining of teeth [4].
In that regard, tooth bleaching is a conservative treatment for tooth stains [5]. This therapy can be performed at home or in the office, with gels containing different hydrogen peroxide (HP) and carbamide peroxide concentrations [6], varying the mode of application and activation with the presence or absence of light [7]. Regardless of the technique or products used, the mechanism of action of bleaching agents is based on a complex oxidation process, with the release of reactive oxygen species that penetrate through the enamel prisms and reach the dentin, breaking down organic molecules and producing smaller and lighter compounds [8].
Some studies [9, 10] recommend that patients undergoing bleaching therapy reduce the intake of coffee, tea, and cigarette consumption, or any other habit that can cause stains on the teeth. However, the literature still presents conflicting results. Other studies claim that the effectiveness of tooth whitening may not be directly affected by diet [11, 12]. This relationship between coloring drinks and bleaching treatment is still a matter of discussion in the literature due to the fact that tooth whitening causes structural changes in the surfaces of teeth, including changes in surface morphology and in the physicochemical properties of enamel [13-15], such as increased superficial porosity, demineralization, degradation of the organic matrix and loss of calcium and phosphate, causing a reduction in surface microhardness, which could increase the susceptibility to dental staining [10, 16].
The perception of tooth color is a complex phenomenon. Its visual assessment can be influenced by several factors [17]. Therefore, it is necessary to carry out a visual analysis using scales or numerical systems to ensure a more universal language and interpretation. When colors are ordered, they can be expressed in terms of hue, lightness and saturation [18]. Thus, to decrease the subjective perception of color, the CIE L * a * b * scale is used to quantify tooth color. The L * a * b * color space was created after the opposite color theory, in which two colors cannot be green and red at the same time, or yellow and blue at the same time. L * indicates brightness and a * and b * are the color coordinates. Spectrophotometers and colorimeters measure light reflected from objects at each wavelength or within specific ranges. It then quantifies the spectral data to determine the object's color coordinates in the L * a * b * color space and presents the information in numerical terms [9]. Thus, it is possible to measure the amount and spectral composition of light reflected, transmitted and absorbed on the tooth surface [19], using a more complete color scale, according to the International Commission on Illumination (CIE) [20].
In-office bleaching systems use HP in high concentrations that can be activated with sources of heat and/or light [21]. The use of light, associated with in-office bleaching, aims to accelerate the bleaching process due to the increase in HP temperature [7]. It is believed that the rise in temperature increases the rate of HP decomposition into free radicals for the oxidation of complex organic molecules [22]. There is a wide variety of light activating sources, such as halogen lamps, lasers, light-emitting diodes (LEDs), metal halide and plasma arc lamps (PACs) [23-25]. However, the role of light activation during the bleaching procedure has been questioned in the literature [26-28].
The goals of the present study were to assess the relationship between tooth bleaching performed with different HP concentrations and surface morphological changes, as well as the predisposition to staining of bleached enamel, assessed through colorimetry, scanning electron microscopy (SEM), and energy-dispersive X-ray spectroscopy (EDS). Additionally, we assessed the effect of using light sources associated with bleaching therapy. The following null hypotheses were considered: H01 - different HP concentrations do not promote color, morphology, and chemical composition changes in bleached enamel; and H02 - light source emission does not promote color, morphology, and chemical composition changes in bleached enamel.
2. MATERIALS AND METHODS
The present study was approved by the Research Ethics Committee of EvandroChagas Institute, Belém do Pará, Brazil, under Opinion No. 021/2009/CEPAN/IEC/SVS/MS. CEPAN Registration No. 0020/2009.
2.1. Specimen Preparation
Bovine incisors were collected and immediately stored in 0.1% thymol solution at 5° C for disinfection. The teeth that exhibited cracks or fractures were discarded. The remaining healthy elements were stored for another 14 days in a disinfectant solution, and were later cleaned. A double-sided diamond disc (Fava, RibeirãoPreto, State of São Paulo, Brazil) was used under the central area of the buccal crown surfaces. Polishing of the pulp face was carried out until it reached a thickness of 3 mm through polishing sandpaper sheets, with pre-determined weights: # 320, 400, 600 and 1000 grain (3M; Sumaré, São Paulo, Brazil), under abundant irrigation to obtain specimens of the same size (6 x 6 x 3 mm). The buccal surface of the incisors was preserved to simulate the same clinical conditions of the bleaching procedure.
2.2. Experimental Group
We obtained twenty-five specimens of bovine incisors, which were divided into five groups (n = 5), according to the concentration and activation or not of LED-laser (Bleaching Laser Light Plus - DMC, São Carlos, State of São Paulo, Brazil). The bleaching treatment was performed for three weeks, with an interval of seven days between each session. All bleaching steps followed the protocols suggested by the manufacturer. The specimens were placed in an Xpress XT silicone matrix (3M do Brasil Ltda., Campinas, State of São Paulo, Brazil) measuring 7 x 7 x 4 cm in order to standardize the 4-mm thickness of the bleaching agent in the sample. Between the bleaching sessions, all specimens were stored in a dark environment immersed in distilled water, in a laboratory incubator, at a temperature of 37° C. Table 1 shows the different experimental groups, type of bleaching treatment, and staining solution.
2.3. Specimen Staining
We used 82.5 grams of ground coffee per liter of water. The solution was heated at 100° C for 30 seconds. The coloring used was composed of cornmeal * enriched with iron and folic acid and oily annatto suspension - soy vegetable oil and natural annatto coloring (Colorífico, Cecim Products, Benevides, Pará Brazil). The staining solution was prepared by heating and adding the coloriferous agent to water heated at 70° C, at a rate of 58.61 grams per liter of oil. Subsequently, the solution was filtered and frozen at -4º C before use. Twenty days after the end of the bleaching process, the specimens were immersed four hours a day for 28 days in coffee and in a stained solution on alternate days.
2.4. Color Measurement
A colorimeter (Colorimeter Tristimulus CR-400, Konica Minolta, NJ, USA) was used to assess color changes. The results of the color measurements were provided by the CIE L*a*b* scale with coordinates L*, a*, and b*. The L* axis represents brightness, which ranges from 0 (black) to 100 (white); the a* axis represents the degree of green (-a*)/red (+a*); and the b* axis represents the degree of blue (-b*)/yellow (+b*). The color was assessed before bleaching (T0), after all the bleaching steps have been conducted (T1), and 28 days after bleaching (T2). The CIE L*a*b* scale data were compared using the parameters ∆L*, ∆a* and ∆b*, and the total color difference (∆E) was calculated using the following equation:
2.5. Scanning Electron Microscopy (SEM)
The specimens were dehydrated initially for seven days. For the following analyses, the specimen was sectioned in half. For SEM analysis, half of the enamel surface area of the samples was coated with gold (Au), using the EMITECH K550x metallizer (Quorum Technologies Ltd., South Stour Avenue, Ashford, Kent, UK), and the other half of the sample was later used for EDS analysis. We used a magnification of 2500 X. The photomicrographs were recorded in a high-resolution digital mode (TIFF format), using the LEO 1450 VP scanning electron microscope (Leo Electron, Ontario, Canada). The double-blind technique was used by three calibrated examiners, who were unaware of which analysis groups the photomicrographs belonged to.
2.6. Energy-dispersive X-ray Spectroscopy (EDS)
Detectable changes in the chemical composition of the teeth, as a result of the whitening treatment, were determined through EDS analysis. The different levels of X-rays of the chemical elements of the sample were detected by an EDS detector of the Gresham brand coupled to the SEM. The analyses were performed on the same samples, using the other half of the specimen, which was not metalized. The MEV configuration was changed to the appropriate geometry for the best detection of the x-ray, a voltage acceleration of 15 kV and a reading time of 30 seconds were used. Using the same operational conditions, five EDS analszes were performed in the different groups studied. The results of both analyses were compared to determine the occurrence of changes.
| Groups | Bleaching Agent | Manufacturer | Batch No. | Whitening Treatment | Staining Solution | Experimental Test |
|---|---|---|---|---|---|---|
| Control | - | No treatment | Coffee Chloriferous |
Colorimetry SEM EDS |
||
| HP35 | Whiteness HP 35% Blue Calcium | FGM, SC, Brazil. | 41010 | 40 minutes of application of the bleaching gel | ||
| HP35+LED | Lase Peroxide Sensy 35% | DMC Ltd, SP, Brazil. | 3505 | 40 minutes of application using Whitening Lase Light Plus (Irradiation for 1 minute. The procedure was performed two more times, with an interval of 3 minutes between applications of light) | ||
| HP20 | Whiteness HP Blue 20% | FGM, SC, Brazil. | 41010 | 50 minutes of application of the bleaching gel | ||
| HP7 | Day White® ACP 7,5% | Discus Dental LLC, Culver City, USA. | 2060 | 1 hour a day of the bleaching gel for 21 days |
| Groups | Mean ± Standart Deviation | ||
|---|---|---|---|
| O | P | Ca | |
| Control | 51,9 ± 4,4a | 18,8 ± 1,4b | 29,3 ± 2,9c |
| HP35 | 48,9 ± 6,1a | 19,7 ± 1,2b | 31,3 ± 3,3c |
| HP35+LED | 50,1 ± 7,8a | 19,2 ± 2,4b | 30,5 ± 5,5c |
| HP20 | 51,5 ± 3,0a | 18,9 ± 1,0b | 29,4 ± 2,0c |
| HP7 | 47,9 ± 6,1a | 20,0 ± 1,9b | 32,0 ± 4,2c |
|
* Different letters represent significant statistical difference; p ≤ 0.05; Tukey test **Equal letters do not reveal statistical significance. |
|||
2.7. Statistical Analysis
The results obtained by colorimetry were assessed using analysis of variance Two Factor ANOVA with Tukey's test (p<0.05) and Pareto Diagram using the STATISTICA 7.0 software (Statistical Package for the Social Sciences, Chicago, USA). We compared the degree of bleaching and staining of the bleaching treatments performed, as well as the influence of photoactivation on the bleaching agent. The data obtained by EDS were submitted to Two Factor ANOVA with Tukey's test (p<0.05) for the assessment of structural enamel loss after bleaching and the use of staining solutions.
3. RESULTS
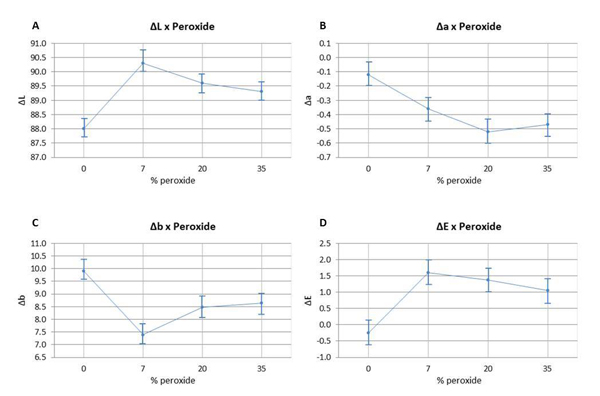
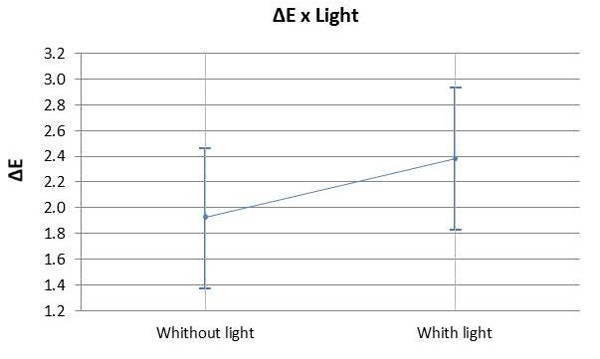
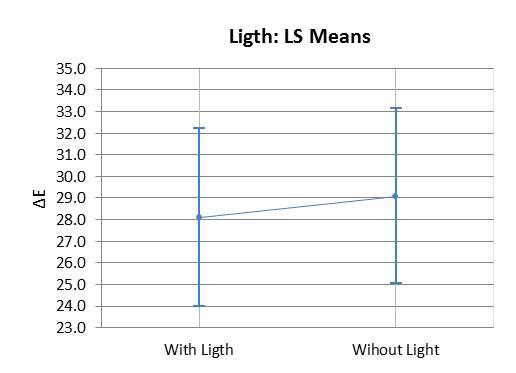
The ANOVA test indicated that the values of ΔL (Fig. 1A) and Δb (Fig. 1C) differed statistically from the values of the other groups with 7.5% HP (p=0.176). There was a significant difference observed between the control group and the bleached groups (p<0.05). The means of Δa (Fig. 1B) and ΔE (Fig. 1/D) did not show any significant difference between the bleached groups (p>0.05). However, the control group differed from the other groups (p<0.05). There was no statistically significant difference (p>0.05) observed between using a light source or not during the bleaching procedure with 35% HP (Graph 1).
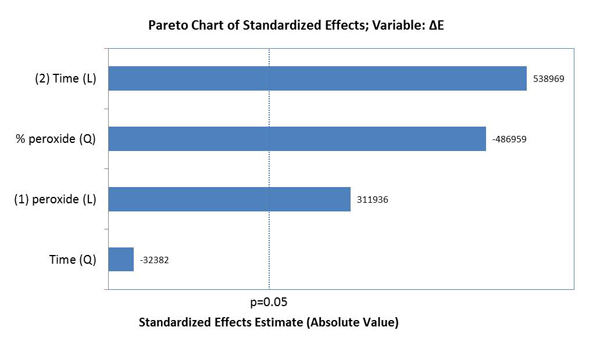
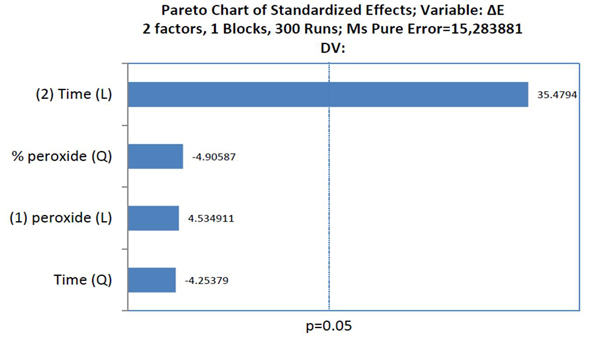
The factors that influenced tooth bleaching determined by the Pareto principle are illustrated in Graph 2. The factors with statistical significance for ΔE were the concentration of HP with a linear and quadratic effect, and time with a linear effect, evidencing that time was the most relevant factor for color variation (p<0.05).
3.1. Specimen Staining
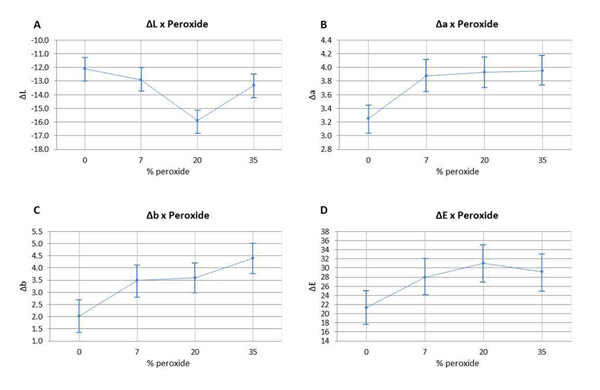
The color variation of the bleached enamel submitted to staining was assessed using ANOVA (p ≥0.05), and can be observed in Fig (2). ΔL, Δa, Δb, and ΔE values are detailed according to the HP concentration. The analysis of ΔL values (Fig. 2A) indicated that the group with 20% HP had a statistically significant difference in comparison to the other groups. The variation of Δa and Δb (Fig. 2B and 2C) had a statistically significant difference between the non-bleached group and the bleached groups; however, there were no significant differences found between the bleached groups. The analysis of ΔE variation (Fig. 2D) did not reveal any statistical difference between the group bleached with 7.5% HP and the control group; however, it did not differ statistically from the other bleached groups with different HP concentrations.
Graph 3 illustrates the assessment of the susceptibility of enamel bleached using 35% HP, with or without a light source during staining. There was no significant difference found in ΔE variation between bleaching with or without light activation (p=0.007). Graph 4 illustrates the variation of ΔE in the specimens subjected to staining with respect to time and HP concentration. It was observed that linear time had a more significant influence on staining than the HP concentrations used.
3.2. Scanning Electron Microscopy
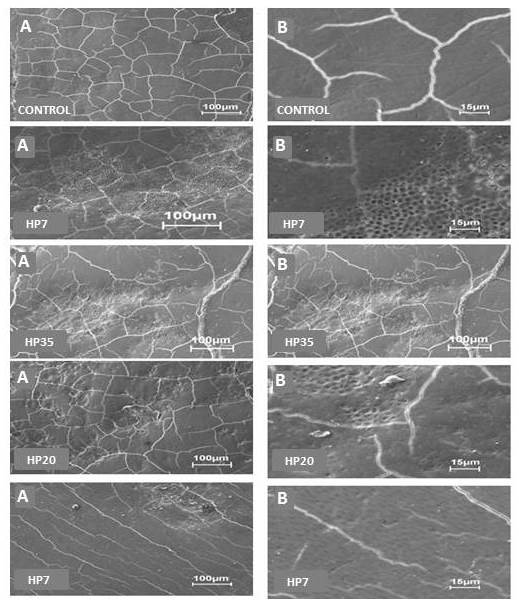
Fig. (3) shows the photomicrographs obtained by SEM. The images were assessed by three examiners using the double-blind method. A shows an increase of 500x, and B an increase of 2500x for each group. In the Control group, the flat enamel displayed only cracks due to dehydration in the SEM analysis. In the HP35 group, the surface of bleached enamel exhibited increased porosity, characterized by the removal of the superficial layer, showing a greater amount and depth of the depressions left by the bleaching process. These areas were isolated, with enamel around them, showing a pattern similar to that of Control. HP35+LED exhibited an increase in porosity and depressions, similar to the changes observed in the HP20 group, however deeper and more extensive. In HP20, the photomicrographs indicated fewer changes in comparison to those of HP35. Irregular areas with less deep porosity were randomly distributed over the surface. The enamel changes promoted in the HP7 group were very discreet, showing removal of the superficial layer, without exposure of enamel prisms.
3.3. Energy-dispersive X-ray Spectroscopy
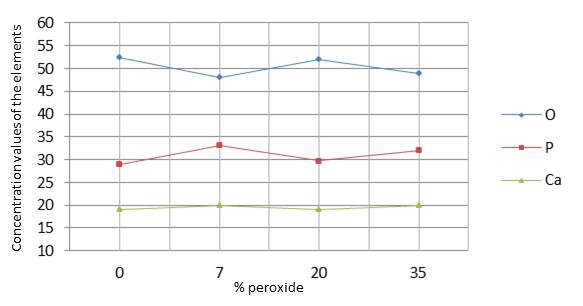
The results in Table 2 indicate that there was no significant difference (p=0.020) between the control group and the groups submitted to bleaching regarding changes in oxygen (O), calcium (Ca), and phosphorus (P). Graph 3 shows the values of oxygen, phosphorus, and calcium on the enamel surface analyzed by ANOVA (p<0.05).
4. DISCUSSION
The CIE L*a*b* three-dimensional colored space was used to represent the perception of colored stimuli. The three characteristic axes are L*, a*, and b*, where L* represents the measure of object brightness, and a* and b* represent the color in the red-green and yellow-blue axes, respectively [19]. Generally, the perception of the bleaching therapy effect results from a decrease in axes a* and b*, and an increase in L* [29].
In the present study, it was possible to observe these changes in the L*, a*, and b* axes. All specimens submitted to different HP concentrations exhibited a significant bleaching response in comparison to Control (Fig. 1). This result was also found in an in vitro study conducted by Al-Omiri et al. [30]. These authors also assessed the bleaching effect of HP, the brightness baseline values with (L*) increased and the b* values decreased, representing the reduction of yellowing (b*), which are considered important factors for tooth bleaching. In addition, it is important to point out that none of the components of the CIE L*a*b* system should be considered in isolation since bleaching is the combination of all tristimulus values (L*, a*, and b*) that are useful to quantify bleaching [19].
The results of the present study also indicated that home bleaching produced a better bleaching effect, since the HP7 group, with the lowest HP concentration (7.5%), showed the best outcomes, being statistically different from the other bleached groups. This finding took into consideration an increase in brightness values and a decrease in yellow-blue values (Δb). This way, H01 was partially rejected, given that the different HP concentrations influenced color change in bovine tooth enamel. The bleaching product used in HP7 had amorphous calcium phosphate, which can cause an increase in the concentrations of these ions in tooth enamel, facilitating tooth surface remineralization [31]. Thus, the incorporation of calcium and phosphate ions in HP7 specimens may have contributed to the optical result observed in this group, since mineral content can influence visual tooth color assessment [17].
The use of light as a source to catalyze the reaction of 35% HP produced the same bleaching result in comparison to conventional chemical activation. Therefore, H02 was accepted, i.e. the presence of light had no influence on color change. Hahn et al. [32] also investigated in vitro the effect of LED on tooth whitening compared to treatments without light activation, although these authors used a higher concentration of hydrogen peroxide (38% HP) than that adopted in the present investigation; it was observed that light activated bleaching agent revealed aesthetic results similar to those of chemically activated bleaching. The literature also presents contradictory results to these findings [33-36]. However, it is worth mentioning that there are great differences regarding the tested lightening methods and light emission protocols, making the comparison between these studies very difficult. On the other hand, a recent meta-analysis study conducted by Maran [7], reinforcing the highest level of scientific evidence, showed that the activation of light regardless of the type of device used for this purpose did not improve the effectiveness of whitening, corroborating the findings of the present investigation.
The results obtained in the present study indicated that the bleached specimens exhibited greater susceptibility to staining in comparison to the specimens of the control group. The HP concentrations used or even the presence of LED light or not did not exhibit a significant difference in terms of predisposition to staining between the bleached groups, thus partially accepting H01. This way, it can be suggested that the changes caused in enamel by the different concentrations of the bleaching agent can favor the accumulation of pigments. Stain susceptibility cannot be related only to surface roughness but to the composition of the enamel and the rate of water absorption, since changes in permeability and irregularities left on the bleached enamel surfaces could facilitate the accumulation of staining agents [5, 37, 38].
It was observed that the time of exposure to pigments was the factor that most changed tooth color. This way, bleaching longevity would mainly depend on the exposure time of the teeth to staining agents. This result is in line with that observed by Côrtes et al. [39], who found statistically significant differences in specimens treated with coffee and wine after bleaching in comparison to the specimens of a control group. Likewise, in another more recent study, Nogueira et al. [12] found that the speed of the bleaching effect had been influenced by the contact with coffee 24 hours after the bleaching sessions, and also that the bleaching outcome had been reversed after one week in all groups.
In this sense, the white diet becomes a point to be investigated with regard to bleaching therapy. Matis et al. [11], in their study, did not observe improvement in the aesthetic result of tooth bleaching associated with a white diet; however, this study did not evaluate additional stains caused by the diet after bleaching, but only during the whitening itself. The same authors also warned that the consumption of dark foods can cause extrinsic stains after the bleaching treatment. Coffee and tea are rich in polyphenols, which have different polarities that provide the yellowish color of these chromogens [40, 41]. This phenomenon can explain the discoloration of the samples observed in the present study after immersion in the staining solutions. These compounds are believed to bind to proteins, such as film or bacteria, on tooth surfaces [40]. In the present experiment, the specimens were immersed for four hours a day, during 28 days, in staining solutions, and stored at a temperature of 37 °C to simulate the effects of a prolonged consumption period. However, the specimens were stored in distilled water, which does not provide the reality of an oral environment. The absence of saliva, tooth brushing, and movements of the tongue and cheeks are limitations of the present study due to the role they play in cleaning tooth surfaces.
It is worth mentioning that the results of published studies on the susceptibility to staining of bleached teeth are still very controversial. And so far, there is no systemic review with meta-analysis that answers this question. Thus, studies that assess the susceptibility to tooth staining of bleached teeth are still needed.
The photomicrographs indicated that different HP concentrations caused morphological changes in bovine enamel, thus partially rejecting H01. Structural changes, such as superficial removal of the aprismatic portion, exposure of enamel prisms, and depressions and grooves were observed in the enamel, which is a result also obtained by Llena [5]. These changes may occur due to the low molecular weight of HP, which is able to penetrate the enamel, affecting its organic phase, and promote a varying degree of porosity on the enamel surface as well as superficial roughness [19].
The factors associated with ultrastructural changes in both enamel and dentin are pH, since products with lower pH generally cause more changes than those of similar concentration, such as neutral or alkaline pH [42], and concentration, i.e. products in high concentrations cause more structural changes than those with low concentrations [43].
The results obtained by EDS indicated that oxygen levels had a small reduction; however, without statistically significant differences between the groups, partially confirming H01, i.e. the different HP concentrations did not influence the chemical composition of bleached enamel. There was an increase in the levels of calcium and phosphorus in the bleached groups in comparison to the control group, without showing a statistically significant difference between the bleached and non-bleached enamel, as also confirmed by Duschner et al. [44] This increase can be explained by the fact that the levels of calcium and phosphorus decrease in dentin and cement, since the organic molecules are attacked by the perhydroxyl radical, which is highly reactive, electrolytic and unstable, releasing calcium and phosphorus ions onto the surface of bleached dental enamel [45]. Eimar et al. [46] also found that HP did not induce significant changes in the relative inorganic content of tooth enamel, and whitened teeth only by oxidizing their organic matrix. These modifications resembled the changes caused by enamel acid etching [47].
CONCLUSION
Based on the results obtained in the present study, it can be concluded that the factors ‘time’ and ‘HP concentration’ influenced color variation in bovine tooth enamel. High concentrations of HP were not necessary to obtain effective bleaching (ΔE). The time of exposure to the staining solution was the main factor for staining bovine enamel. In all groups, HP caused superficial morphological changes in the enamel; however, it was not able to cause significant changes in the content of oxygen, calcium, and phosphate ions on the enamel surface. The light source had no effects on tooth bleaching.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The present study was approved by the Research Ethics Committee of Evandro Chagas Institute, Belém of Pará, Brazil, under Opinion No. 021/2009/CEPAN/IEC/SVS/MS. CEPAN Registration No. 0020/2009.
HUMAN AND ANIMAL RIGHTS
The authors followed the standards established by The US National Research Council's "Guide for the Care and Use of Laboratory Animals".
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The authors confirm that the data supporting the findings of this research are available within the article.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflicts of interest, financial or otherwise.
ACKNOWLEDGEMENTS
This study was supported by infrastructural maintenance by the Coordination for the Improvement of Higher Education Personnel (CAPES) and the Federal University of Pará (UFPA).


