Use of Lip Protecting Agents in the Prevention of Actinic Cheilitis, Herpes Labialis and Cancer of Lip: A Systematic Review
Abstract
Background:
Actinic cheilitis, herpes labialis and lip cancer are relatively common conditions presenting on the lips associated with exposure to periods of sun exposure and thereby ultraviolet radiation.
Objective:
This systematic review aimed to determine the efficacy of the application of sunscreen-containing lip-protecting agents (LPA) in the prevention of actinic cheilitis (AC), recurrent herpes labialis (RHL) and lip cancer (LC).
Methods:
This review was conducted in accordance with the PRISMA guidelines and registered with the PROSPERO database. A literature search was conducted using SCOPUS, Google Scholar, Medline (Ovid), Pubmed, CINAHL, Cochrane Library databases and manual search using search terms actinic cheilitis (AC), recurrent herpes labialis (RHL) and lip cancer (LC) along with lip protecting agents and their variations as keywords. A total of 1,567 papers were yielded. Of them, nine studies were eligible for qualitative data synthesis.
Results:
Nine articles (3 AC, 5 RHL, 1 LC) were deemed eligible and thus selected for qualitative synthesis. Three studies on AC identified approximately 21.7% lower prevalence of lesions when some form of lip protection was used. Eighty percent of studies on RHL identified that the application of LPA is effective in preventing RHL. Subjects who applied LPA more than once daily only had half the risk of having LC compared to those who applied once daily.
Conclusion:
This review of randomised controlled trials (RCTs) and observational studies supports the use of LPA as an effective method in preventing lip-associated lesions. Further, RCTs and observational studies should aim at determining a definitive LPA application regime and optimal SPF strength to prevent lip-associated lesions.
This systematic review was registered with the International Prospective Register of Systematic Reviews (PROSPERO): Registration Number - CRD42020177484. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020177484
1. INTRODUCTION
Ultraviolet Radiation (UVR) is found in abundance in the environment, contributing to a spectrum of skin lesions including inflammatory lesions, degenerative aging and skin cancers [1]. Actinic cheilitis, herpes labialis and lip cancer are relatively common conditions presenting on the lips that can cause considerable morbidity, and less frequently, mortality [2]. The common major etiological factor for those lip lesions is exposure to ultraviolet radiation. Those who experience long periods of sun exposure, often due to occupational or recreational reasons, are vulnerable to the harmful effects of UVR [1]. Yet, the lips are frequently neglected when it comes to sun protection [1]. Knowledge from existing studies [3, 4] of the lip lesions predominantly surrounds the pharmacological aspect of lesion resolution. However, to date, there is a knowledge gap in the aspects concerning prevention.
Actinic Cheilitis (AC) is a potentially malignant oral disorder that frequently affects the vermillion border of the lower lip, with a global prevalence of 0.45% to 2.4% [5]. UVR is the predominant etiological factor for AC development. A variety of premalignant lesions are found in patients exposed to UVR, including AC. Ultraviolet A and B promote local immunosuppression and changes in proteins, contributing indirectly to dysplastic changes. The production of reactive oxygen species induced by UVA contributes to oxidative stress, which causes cellular damage that leads to the onset of AC [2]. Clinically, the lower lip often displays scales, fissures and erosions [6]. In advanced disease, blurred demarcation between the lip vermillion border and skin is observed, in addition to atrophic, erosive or keratotic plaques [6, 7]. The keratotic patches may progressively thicken and ulcerate, that are suggestive of malignant transformation [6, 7]. AC occurs more frequently in those with fair complexions, males, the elderly, those who live at high altitudes, and most significantly, those with high exposure to the sun [8-10]. Other factors linked to the malignant transformation of AC include tobacco smoking and genetic predisposition [8-10].
Although some contributing factors related to AC, such as skin complexion and occupation, may be unmodifiable, sunscreen-containing LPAs should still be applied due to their known prophylactic and alleviating effects on AC [7]. Savage et al. have outlined a prevention protocol for AC. This includes limiting sun exposure, wearing a broad-brimmed hat and application of a broad-spectrum sunscreen with a Sun Protection Factor (SPF) higher than 30 applied every two hours when outdoors [11]. Despite UVR being the principal cause of AC, there have been limited reports on the effectiveness of sunscreen in the prevention of AC. Yet, its analogous counterpart affecting the skin, actinic keratoses, has been widely studied [12-14].
Herpes labialis, commonly known as “cold sore”, is the reactivation of viral infection caused by Herpes Simplex Virus (HSV) type 1 that manifests primarily on the lips and perioral region [15]. HSV is mainly transmitted through oral-to-oral contact with a virus-shedding individual [16]. It is estimated that around 16% to 45% of the global population has been infected with HSV [17]. Once acquired, it can cause herpetic gingivo-stomatitis and remains dormant in nerve tissues to reactivate as Recurrent Herpes Labialis (RHL) at any age [18]. RHL lesions can be presented in grouped vesicles and erythema, causing discomfort or pain [19]. It has an average incidence of 1.6 and an average prevalence of 2.5 per 1,000 population annually [18]. About one-third of patients with a history of RHL will experience at least one recurrence in their lifetime [20]. Recurrence can be induced by a variety of intrinsic and extrinsic factors, including exposure to ultraviolet radiation, high fever, trauma, emotional stress and immune suppression [18].
Studies have been conducted on herpes labialis but most of them focused on the pharmacological aspect of treatment, such as the efficacy of different types and forms of antiviral medications [21] Potential genetic components that may influence the recurrence of herpes labialis have also been studied [13]. However, there are a limited number of studies exploring the effect of ultraviolet radiation independently on Recurrent Herpes Labialis (RHL). Most of the studies utilised sunscreen-containing LPAs as a placebo in their studies without investigating the potential protective effect of LPAs.
Lip cancer (LC) has a prevalence of 12 per 100,000 and an incidence of 4 per 100,000 [22]. The vermilion border of the lips, especially the lower lip can be directly exposed to sunlight, and hence carries a high risk of developing lip cancer [23]. The main histological types of LC are squamous cell carcinoma and basal cell carcinoma [24]. As squamous cell carcinoma is strongly related to cumulative constant UV exposure, LPAs with UV barriers are of importance in preventing the occurrence of lip cancers [25].
The objective of this systematic review is to analyse and critically appraise current literature to determine the effectiveness of sun protection factor containing LPAs (commercial and test formulation) in the prevention of actinic cheilitis, herpes labialis and lip cancer lesions.
2. METHODS
2.1. Protocol and Registration
This systematic review was registered with the International Prospective Register of Systematic Reviews (PROSPERO): https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020177484 (registration number CRD42020177484). The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used to conduct this review [26].
2.2. Search Strategy and Selection Criteria
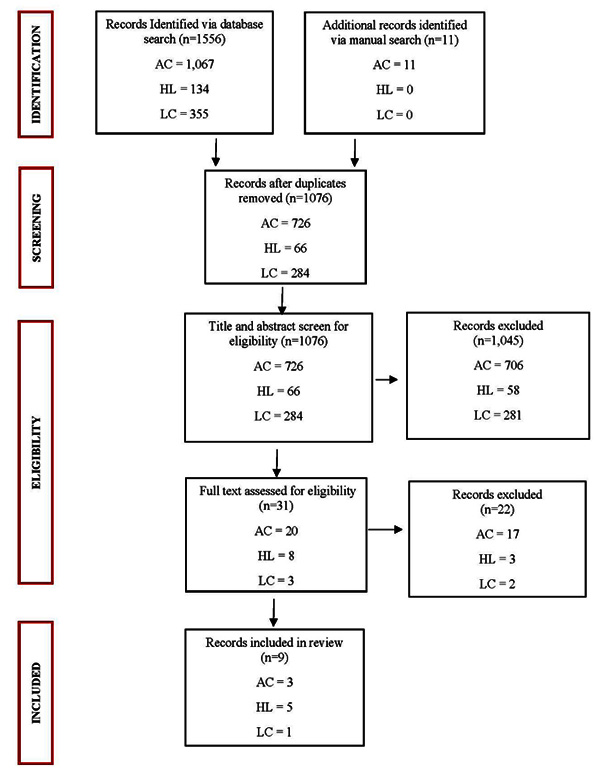
A comprehensive literature search for studies of each lip condition (AC, RHL, LC) was conducted using the following electronic databases: SCOPUS, Google Scholar, Medline, PubMed, The Cumulative Index to Nursing and Allied Health Literature (CINAHL), and the Cochrane Library on 4 April 2020. A full list of the search terms used for each lip condition in each database is provided in Table (1). A manual search for articles was also conducted. Predetermined inclusion and exclusion criteria were used to filter out studies relevant to our review. The inclusion criteria were human studies published in English language, full-text availability, peer-reviewed, randomised controlled trials, observational studies, and focusing on the prevention of lip-associated lesions through the use of an LPA. The exclusion criteria were articles published in other languages than English, full-text unavailable, a non-peer review, case reports, idea, editorial and anonymous survey, use of LPA containing an antiviral, and animal studies. No limit was placed on the date of publication of the articles. A total of two studies (2 AC, 0 RHL, 0 LC) were excluded due to inaccessible full-text. Of the studies selected, duplicates were removed, and the remaining studies were reviewed for relevance according to title, abstract and full-text, as illustrated in (Fig. 1). Articles that were excluded after full-text screening did not mention lesions occurring on the lips specifically and did not report the prevalence of the lip lesions. Articles that did not concern lip protection but general sun protection only were also excluded.
| Database | Lip Condition and Search terms | ||
| RHL | AC | LC | |
| Scopus | (sunscreen OR sunblock OR “skin protection” OR “sun protection” OR “UV protection” OR “sun filter” OR photoprotect* OR “sun tan lotion” OR “lip balm” OR “lip care” OR lotion or “lip cream” or lipstick) AND (“cold sore” OR “herpes labialis” OR “herpes simplex labialis” OR “oral herpes” OR “herpetic gingivostomatitis” OR “Recurrent gingivostomatitis” OR “recurrent herpes” OR “fever blisters”) |
(sunscreen OR sunblock OR “skin protection” OR “sun protection” OR “UV protection” OR “sun filter” OR photoprotect* OR “sun tan lotion” OR “lip balm” OR “lip care” OR lotion or “lip cream” or lipstick) AND (“solar cheilitis” OR “actinic cheilitis” OR “solar cheilosis” OR “actinic cheilosis” OR “actinic keratosis” OR “actinic keratosis” OR “solar keratosis” OR “sailor’s lip” OR “farmer’s lip” OR “farmer lip”) |
(lip OR labial OR mouth) AND sunscreen OR sunblock OR (“skin protection” OR “sun protection” OR “UV protection” OR “sun filter” OR photoprotect* OR “sun tan lotion” OR “lip balm” OR “lip care” OR lotion or “lip cream” or lipstick) AND (cancer OR neoplasm OR malignan* OR carcinoma) |
| Medline | Radiation-Protective Agents OR Sunscreening Agents OR sunblock OR skin protection OR sun protection OR UV protection OR sun filter OR photoprotection OR sun tan lotion OR lip balm OR lip care OR lotion OR lip cream OR lipstick AND Herpes Labialis OR cold sore OR herpes simplex labialis OR oral herpes OR herpes gingivostomatitis OR Recurrent Herpes Gingivostomatitis OR fever blisters | Radiation-Protective Agents OR Sunscreening Agents OR sunblock OR skin protection OR sun protection OR UV protection OR sun filter OR photoprotection OR sun tan lotion OR lip balm OR lip care OR lotion OR lip cream OR lipstick AND Cheilitis OR solar cheilitis OR actinic cheilitis OR solar cheilosis OR actinic cheiloses OR Keratosis, Actinic OR actinic keratosis OR solar keratosis OR solar keratosis OR sailors lip OR farmers lip OR farmer lip | Lip OR labial OR Mouth AND Radiation-Protective Agents OR Sunscreening Agents OR sunblock OR skin protection OR sun protection OR UV protection OR sun filter OR photoprotection OR sun tan lotion OR lip balm OR lip care OR lotion OR lip cream OR lipstick AND cancer OR neoplasms OR carcinoma OR malignancy |
| Pubmed | sunscreen OR sunblock OR “skin protection” OR “sun protection” OR “UV protection” OR “sun filter” OR photoprotect* OR “sun tan lotion” OR “lip balm” OR “lip care” OR lotion or “lip cream” or lipstick AND “cold sore” OR “herpes labialis” OR “herpes simplex labialis” OR “oral herpes” OR “herpetic gingivostomatitis” OR “Recurrent gingivostomatitis” OR “recurrent herpes” OR “fever blisters” |
sunscreen OR sunblock OR “skin protection” OR “sun protection” OR “UV protection” OR “sun filter” OR photoprotect* OR “sun tan lotion” OR “lip balm” OR “lip care” OR lotion or “lip cream” or lipstick AND “solar cheilitis” OR “actinic cheilitis” OR “solar cheilosis” OR “actinic cheilosis” OR “actinic keratosis” OR “solar keratosis” OR “sailor’s lip” OR “farmer’s lip” OR “farmer lip” | sunscreen OR sunblock OR “skin protection” OR “sun protection” OR “UV protection” OR “sun filter” OR photoprotect* OR “sun tan lotion” OR “lip balm” OR “lip care” OR lotion or “lip cream” or lipstick AND lip OR labial OR mouth AND cancer OR neoplasm OR malignan* OR carcinoma |
| Cochrane | “cold sore” OR “herpes labialis” OR “herpes simplex labialis” OR “oral herpes” OR “herpetic gingivostomatitis” OR “Recurrent gingivostomatitis” OR “recurrent herpes” OR “fever blisters” in Title Abstract Keyword AND "sunscreen" OR "sunblock" OR “skin protection” OR “sun protection” OR “UV protection” OR “sun filter” OR photoprotect* OR “sun tan lotion” OR “lip balm” OR “lip care” OR "lotion" OR “lip cream” or "lipstick" in Title Abstract Keyword - (Word variations have been searched) | “solar cheilitis” OR “actinic cheilitis” OR “solar cheilosis” OR “actinic cheilosis” OR “actinic keratosis” OR “actinic keratosis” OR “solar keratosis” OR “sailor’s lip” OR “farmer’s lip” OR “farmer lip” in Title Abstract Keyword AND "sunscreen" OR "sunblock" OR “skin protection” OR “sun protection” OR “UV protection” OR “sun filter” OR photoprotect* OR “sun tan lotion” OR “lip balm” OR “lip care” OR "lotion" OR “lip cream” or "lipstick" in Title Abstract Keyword - (Word variations have been searched) |
"lip" OR "labial" OR "mouth" in Title Abstract Keyword AND "cancer" or "neoplasm" OR malignan* OR carcino* in Title Abstract Keyword AND "sunscreen" OR "sunblock" OR “skin protection” OR “sun protection” OR “UV protection” OR “sun filter” OR photoprotect* OR “sun tan lotion” OR “lip balm” OR “lip care” OR "lotion" OR “lip cream” or "lipstick" in Title Abstract Keyword - (Word variations have been searched) |
| Cinahl | lip OR labial OR mouth OR (MH "Lip") AND sunscreen OR sunblock OR “skin protection” OR “sun protection” OR “UV protection” OR “sun filter” OR photoprotect* OR “sun tan lotion” OR “lip balm” OR “lip care” OR (MH "Sunscreening Agents") AND “cold sore” OR “herpes labialis” OR “herpes simplex labialis” OR “oral herpes” OR “herpes gingivostomatitis” OR “Recurrent gingivostomatitis” OR “recurrent herpes” OR “fever blisters” | lip OR labial OR mouth OR (MH "Lip") AND sunscreen OR sunblock OR “skin protection” OR “sun protection” OR “UV protection” OR “sun filter” OR photoprotect* OR “sun tan lotion” OR “lip balm” OR “lip care” OR (MH "Sunscreening Agents") AND “solar cheilitis” OR “actinic cheilitis” OR “solar cheilosis” OR “actinic cheiloses” OR “actinic keratosis” OR “actinic keratoses” OR “solar keratosis” OR “solar keratoses” OR “sailor’s lip” OR “farmer’s lip” OR “farmer lip” OR (MH "Keratosis, Actinic") OR (MH "Cheilitis") | lip OR labial OR mouth OR (MH “Lip”) AND sunscreen OR sunblock OR “skin protection” OR “sun protection” OR “UV protection” OR “sun filter” OR photoprotect* OR “sun tan lotion” OR “lip balm” OR “lip care” OR (MH “Sunscreening Agents”) AND cancer OR neoplasm OR “malignan*” OR carcinoma OR (MH “Lip Neoplasms”) |
| Google Scholar | sunscreen OR sunblock OR “skin protection” OR “sun protection” OR “UV protection” OR “sun filter” OR photoprotect* OR “sun tan lotion” OR “lip balm” OR “lip care” OR lotion or “lip cream” or lipstick OR Radiation-Protective Agents OR Sunscreening Agents AND “cold sore” OR “herpes labialis” OR “herpes simplex labialis” OR “oral herpes” OR “herpetic gingivostomatitis” OR “Recurrent gingivostomatitis” OR “recurrent herpes” OR “fever blisters” | sunscreen OR sunblock OR “skin protection” OR “sun protection” OR “UV protection” OR “sun filter” OR photoprotect* OR “sun tan lotion” OR “lip balm” OR “lip care” OR lotion or “lip cream” or lipstick OR Radiation-Protective Agents OR Sunscreening Agents AND “solar cheilitis” OR “actinic cheilitis” OR “solar cheilosis” OR “actinic cheilosis” OR “actinic keratosis” OR “solar keratosis” OR “sailor’s lip” OR “farmer’s lip” OR “farmer lip” | sunscreen OR sunblock OR “skin protection” OR “sun protection” OR “UV protection” OR “sun filter” OR photoprotect* OR “sun tan lotion” OR “lip balm” OR “lip care” OR lotion or “lip cream” or lipstick OR Radiation-Protective Agents OR Sunscreening Agents AND lip OR labial OR mouth AND cancer OR neoplasm OR malignan* OR carcinoma |
2.3. Qualitative Synthesis
Articles selected for full-text review were distributed between two groups of members. SM, MR and AVS analysed the first half of the articles, and AS and ST reviewed the second half of the articles. The inclusion and exclusion criteria were used to select studies relevant for review. An electronic Microsoft Excel spreadsheet was created to extract data from the studies following the PICOS framework (population, intervention, comparison, outcomes and study type), along with the type of lip-protective agent used (Table 2). When disagreement arose between two reviewers, a third reviewer was consulted and a consensus was reached. The final analysis of the data was verified by each author (SM, MR, AVS, AS, ST) and was reaffirmed by the senior authors (AA, DS).
| Actinic cheilitis | |||||
| Author. Year. | Location | Study Type | Population | Intervention/Lip Product Studied | Measured Outcome |
| de Oliveira RA, da Silva L, et al. 2014. | Brazil | Cross-sectional | 210 randomly selected Brazilian fishermen and women. | N/A | Prevalence of AC with sun block was 0%. Prevalence of AC without sunscreen was 21.7% (estimate obtained from regression model). |
| de Oliveira BH, Gonzaga A, et al. 2019 | Unknown | Randomised Controlled Trial | 23 subjects with AC. | Fludroxycortide and Lip sunscreen | LS only patients presented 1 with complete improvement, 4 with partial improvement and 3 with no change. F+LS patients- 5 with total remission, 7 with partial improvement, 3 with no changes. |
| dos Santos R, de Oliveira R, et al. 2018. | Brazil | Cross-sectional study | 201 male subjects. | N/A | 57.9% had AC with lip protection. 36.2% had AC without lip protection. |
| Recurrent Herpes labialis | |||||
| Author. Year. | Location | Study Type | Population | Intervention/Lip Product Studied | Measured Outcome |
| Duteil L, Queille-Roussel C, et al. 1998. | Unknown | Randomised crossover study | 19 subjects (11 females, 8 males). | A sunblock stick containing Mexoryl SX, Eusolex 6300 and Parsol 1789. | 10 out of 19 subjects had RHL after using vehicle but no RHL after sunblock. |
| Mazzarello V, Ferrari M, et al. 2019. | Sardinia, Italy | Randomized crossover study | 20 adult subjects (8 males, 12 females) serving as their own controls. | Sun protecting lipstick with SPF 30 for 30 days. Application every 2 hours, after eating, drinking, smoking, and swimming | 1/20 developed RHL with sunblock stick. 10/20 developed RHL without sunblock stick. |
| Mills J, Hauer L, et al. 1987. | United States | Randomised Controlled Trial | 51 subjects (31 males, 20 females) with a history of recurrent orofacial herpes triggered by skiing. | Sunscreen (lipstick and lotion form) with SPF 15. Either UVA or UVB sunscreen-containing PABA and benzophenone. Applied hourly before skiing. | 3/24 subjects had reoccurrence in sunscreen group. 3/27 patients had reoccurrence in placebo group. |
| Rooney J, Bryson Y, et al. 1991. | United States | Randomized crossover trial | 38 subjects (30 females and 8 males) with a history of recurrent herpes labialis at least once per year and who were seropositive for HSV. | Commercially available Sunscreen with SPF 15 (Total Eclipse AB) | With placebo and UV, 27/38 (71%) developed RHL. With sunscreen and UV, no RHL developments were observed, except 1/35 shed virus at exposure site. |
| Shulman J, Carpenter W, et al. 1992. | United States | Observational and Interview | 1,062 subjects (1,035 males, 27 females) | Army-issued wax stick type lip protectant and commercially available lip protectants (not specified) | RHL in 4% of subjects with prevalence rates as high as 10% of soldiers not using lip protectants. Lip protectant reported by 77% of subjects, with non-users having 4 times higher prevalence of RHL than users. |
| Lip Cancer | |||||
| Author. Year. | Location | Study Type | Population | Intervention/lip product studied | Measured outcome |
| Pogoda and Preston-Martin S. 1996. | United states | Observational (population-based case-control study) | 74 females with lip cancer, 105 controls | Lip protectant covering | Subjects with higher exposure to average UVR flux (at or above 430) with less than one application of lip protection a day had the highest risk of LC. |
2.4. Assessment of Risk of Bias in Individual Studies
The risk of bias and level of evidence for each included study was assessed by two of the reviewers (SM, AS, AVS, MR, ST) independently. Differences in judgment were resolved through mediation by a third reviewer. To ensure reliability of the results, the final results were checked through a group discussion involving all members of the group until a consensus was reached. The Cochrane Collaboration tool for assessing the risk of bias was used for Randomised Controlled Trials (RCT) and ROBINS-I (Risk Of Bias In Non-randomised Studies- of Interventions) was used for non-randomised studies [27-29]. The Cochrane Collaboration tool evaluates six sources of bias: Random Sequence Generation, Allocation Concealment, Blinding of Participants and Personnel, Blinding of Outcome Assessment, Incomplete Outcome Data, Selective Reporting [27]. In ROBINS-I, sources of bias were divided into 3 domains: pre-intervention, at intervention and post-intervention [28]. Each domain was assessed individually for low, moderate, serious or critical risk [28]. Results of all domains were then analysed to determine the overall risk of bias for the specific study [28]. The risk of bias of each study was summarised and tabulated (Table 3).
3. RESULTS
A total of 1,567 articles were identified via databases (1556 articles) and manual searches (11 articles) (Fig. 1). Articles were screened in the order of title, abstract, and full text. Duplicate articles were removed, thus yielding 1076 articles for eligibility screening. Nine (3 AC, 5 RHL, 1 LC) articles were deemed eligible and thus selected for qualitative synthesis (Table 2).
| Study | Pre-Intervention | At Intervention | Post-Intervention | Overall Risk of Bias | ||||
| First Author. Year | Bias Due to Confounding | Bias in Selection of Participants Into the Study | Bias in Classification of Interventions | Bias Due to Deviations from Intended Interventions | Bis Due to Missing Data | Bias in Measurement of Outcomes | Bias in Selection of the Reported Result | Low / Moderate / Serious / Critical |
| de Oliveira Ribeiro, A. 2014 | Low | Low | Low | Low | Moderate | Low | Low | Moderate |
| dos Santos, R. 2018 | Low | Low | Low | Low | Low | Low | Low | Low |
| Shulman, J. 1992 | Low | Moderate | Serious | Moderate | Low | Moderate | Low | Serious |
| Pogoda, J. 1996 | Low | Low | Low | Low | Low | Low | Low | Low |
Five studies related to RHL were included for review [30-34]. All studies assessed some form of LPA in the prevention of RHL. Mills et al. [30], Rooney et al. [32], Mazzarello et al. [34], and assessed the use of a sunscreen-containing LPA in the prevention of RHL. Duteil et al. [31] studied a form of LPA in the prevention of RHL, however, the SPF of the product was not stated. Similarly, Shulman et al. [33] did not state the SPF in the LPA used. There was a significant variation across the studies in terms of the number of participants used. All studies, except Shulman et al. [33], which was an observational study, consisted of a control group. The control group in the Duteil et al. [31] was given a lip protectant without sunlight-absorbing filters. Mills et al. [30] and Rooney et al. [32] provided a placebo to their control groups. The participants in the study conducted by Mazzarello et al. [34] posed as both the study and control group. In the study conducted by Shulman et al. [33], the control group received no LPA. All studies, except Shulman et al. [33], conducted a follow-up or review with their study participants either in the form of a questionnaire or clinical exam.
Three articles related to AC were included for review. In the studies conducted by de Oliveira Ribeiro et al. [2] and dos Santos et al. [35], the prevalence of AC was conducted amongst a group of participants. The participant size of both studies consisted of greater than 200 participants. In these studies, however, it was not specified whether follow-up appointments were conducted and what type of LPA was used. The study conducted by de Oliveira Bezerra et al. [21], which consisted of 23 participants, assessed the efficacy of fludroxycortide, a topical corticosteroid, combined with lip sunscreen in the treatment of AC [21]. In this particular study, there was also a control group that was treated with lip sunscreen alone. Follow-ups were conducted weekly [21].
Only a single study related to LC was deemed eligible for inclusion in this review. The study conducted by Pogoda et al. [36] was an observational study, which explored the hypothesis that the incidence of lip cancer was greater in men partly due to the frequent use of lip protection by women. This study was conducted in the United States in 1996 and consisted of 74 women diagnosed with lip cancer and a control group of 105 participants [36]. The study was conducted via an interview or verbatim questionnaire [36]. The type of LPA studied was not stated as the main indication was to assess LC prevalence in women due to frequent wear of lip covering, a potential protecting behaviour against sun exposure [36]. No follow-up or reviews were conducted [36].
| - | De Oliveira Bezerra, 2019 | Duteil, 1998 | Mazzarello, 2019 | Mills, 1987 | Rooney, 1991 |
| Randomised sequence generation (selection bias) | ? | ? | + | + | + |
| Allocation concealment (selection bias) | ? | ? | ? | + | ? |
| Blinding of participants and personnel (performance bias) | + | + | + | + | + |
| Blinding of outcome assessment (detection bias) | + | + | + | + | + |
| Incomplete outcome data (attrition bias) | - | + | + | + | + |
| Selective reporting (reporting bias) | + | + | + | + | + |
| Other bias | + | + | + | - | + |
3.1. Risk of Bias in Included Studies
The Cochrane Collaboration tool for assessing the risk of bias and ROBINS-I were used to determine the risk of bias and evidence quality for RCTs and observational studies respectively. The risk of bias of each study was summarised and tabulated (Tables 3 and 4).
Only two observational studies of the nine overall included studies resulted in a low risk of bias across all domains [35, 36]. However, no study was deemed of high enough risk of bias to be excluded from this systematic review.
3.2. Quality Assessment of Included Studies
An overall low quality of evidence implies that the study was excluded from the review. Moderate overall gradings were given to AC and RHL, however, due to lack of information, grading could not be performed for LC.
GRADE assessment demonstrated that all included observational studies and RCTs exhibited a moderate quality of evidence. The observational studies were automatically downgraded due to weaker evidence compared to RCTs. Evidence from other studies was predominantly downgraded due to risk of bias, most significantly relating to lack of blinding of participants or outcome assessors. Heterogeneity across the combined studies of AC and RHL was moderate, however, LC studies could not be assessed against this factor. Failure to calculate sample sizes and inadequate sample sizes were also major contributors resulting in the downgrading of evidence.
3.3.1. Recurrent Herpes Labialis (RHL)
Five articles related to RHL satisfied our inclusion criteria [30-34]. They examined the prevalence of RHL with the use of LPA. All studies involved patients with a history of at least one RHL recurrence in a year.
Mills et al. [30] conducted an RCT with a high level of evidence on 51 volunteers in 3 ski resorts in the United States (41 men and 10 women). The participants applied either an LPA with a lotion containing SPF 15 (n=24) or a placebo (n=27) while being observed for one week between January and April 1984. They reported a 12% prevalence of RHL (6 of 51 subjects), equally divided between the test group and control group. The authors concluded that the LPA was not effective in preventing RHL [27].
Duteil et al. [31] conducted a hospital-based, two-way crossover study with high level of evidence in France in 1998. All 19 participants (8 men and 11 women) applied a sunscreen-containing LPA in and its inactive vehicle without UV filters respectively in 2 phases of the study, which were performed at a 4-week interval. Only 5.25% of participants (1 of 19 subjects) had RHL with LPA, whereas 57.9% (11 of 19 subjects) had RHL with the placebo [31]. It was concluded that the use of LPA has a significant benefit in preventing RHL [31].
Rooney et al. [32] conducted a hospital-based double-blinded crossover trial with a high level of evidence in 1991. Of the 38 participants (30 women and 8 men, 37 Caucasians and 1 black), only 35 of them participated in both phases of the trial. In the first phase of using placebo (n=38), the recurrence rate of RHL was 71% (27 subjects) while the recurrence rate was only 3% (1 subject) in the second phase of applying LPA with an SPF 15 (n=35). All participants were followed up for six months after study completion [32]. It was also found that the chance of reactivation of RHL is independent of the previous history of recurrence [32]. The authors concluded that the application of an LPA can effectively reduce the recurrence rate of RHL [32].
Shulman et al. [33] performed a two-week observational study on 1,062 arm personnel (1,035 men and 27 women) of moderate level of evidence in the Mojave Desert, the United States in September 1983. The prevalence of RHL was 10% in those who did not use LPA, which was estimated to be four times higher than LPA users [33]. However, the participants applied either army-issued LPA, commercially available LPA which were available with and without a UV filter, or no LPA [33]. This introduced a serious limitation to the study as LPA used was not uniform and it was unclear whether they contained the same level of UV protection. There may have been impacts of confounding factors on the results, such as the anti-desiccant effects of the LPAs. It was concluded that LPAs containing UV filters reduce the risk of RHL [33].
Mazzarello et al. [34] conducted a randomised crossover trial with a high level of evidence, involving 20 participants (8 men and 12 women) between May and July 2017 in Sardinia, Italy, as they performed daily activities for one month with an LPA (SPF 30) or no protection [34]. Without the use of LPA, 50% (10 subjects) had RHL while only 5% (1 subject) had RHL. It was concluded that the use of LPA was effective in RHL prevention [34].
Based on the above studies, the application of LPA provides some protection in the prevention of RHL. However, the quality of the evidence is insufficient such that considering the relatively lower SPF of the LPA used and the narrow demographic of the participants, it is questionable whether the results can be generalised to a larger population. More good quality RCTs are required to ascertain the efficacy of the LPA for the prevention of RHL.
3.3.2. Actinic Cheilitis (AC)
Three articles related to AC were included in our review [2, 21, 35]. The studies included examined the prevalence of AC with the use of LPA. However, inter-study variation existed when determining the use of LPA or administering LPA.
De Oliveira Ribeiro et al. [2] conducted a cross-sectional observational study in Brazil among a group of fishermen and women to determine the prevalence of AC. The use of sunscreen-containing LPA was observed, and findings revealed 0% prevalence of AC with the use of LPA and 21.7% prevalence in non-LPA users [2]. Moreover, the researchers estimated a probability of 55% for AC with non-use of LPA and found that this probability decreased to 35% when LPA was used [2].
Dos Santos et al. [35] conducted a cross-sectional observational study in Brazil among extractive mining workers to determine the prevalence of AC. The information gathered pertained to the use of sun protection [35]. These forms of sun protection included a hat, a palhoca (improvised individual protection made of straw constructed in the workplace), and LPA [35]. Dos Santos et al. found there was a prevalence of 38.4% of AC among those with some form of sun protection and a 45.5% prevalence of AC among those with no protection [35]. However, when LPA alone was assessed, they found a 57.9% prevalence of AC with LPA use and a 36.2% prevalence of AC when no LPA was used [35]. However, these results were not statistically significant [35].
De Oliveira Bezerra et al. [21] conducted a comparative RCT among a group of 23 patients who were clinically and/or histopathologically diagnosed with AC to analyse the efficacy of Fludroxycortide 0.125mg/g in the remission of AC signs and symptoms. An LPA with an SPF of 60 was used in combination with Fludroxycortide in the test group of 15 participants. The control group consisted of 8 participants and used the same LPA as in the test group. A weekly follow-up was conducted. The control group findings revealed one patient presented total remission of the lesion characteristics of AC, four demonstrated partial improvement, three exhibited no clinical lip alterations, and no cases presented worsening [21]. Although the participants in this study already presented with AC before intervention and control, it is important to note that LPA can be effective in the remission of some AC lesions [21].
Based on the above studies, the application of LPA provides some protection in the prevention of AC. However, further good quality RCTs are required to ascertain the efficacy of LPA for the prevention of AC.
3.3.3. Lip Cancer (LC)
Pogoda et al. [36] used a verbatim questionnaire to ascertain information relating to the risk of LC, including the use of LPA. To investigate the relationship between the use of LPA and the incidence of LC, individuals were divided based on average UV flux [36]. Pogoda et al. found that those in the high exposure group with an average UV flux of 430 or greater who applied LPA no more than once a day had twice the risk of having LC (OR=7.3) compared to those who applied LPA more than once a day (OR=3.2) [36].
4. DISCUSSION
The studies included demonstrated that sunscreen-containing LPAs are effective in preventing the occurrence and progression of these lesions. This review was hence conducted to compile the available evidence on the effectiveness of such agents.
In three of the included studies for RHL, more than 50% of the participants who did not use LPA developed recurrence of RHL, compared to less than 5% in those who used LPA, after receiving the same amount of UVR exposure [31, 32, 34]. There was high-quality evidence supporting that appropriate use of LPA prevents recurrence of HL in studies by Mills et al. and Rooney [30, 32].
However, despite the evidence supporting the use of lip protectants in preventing RHL, interstudy variations make the generalisation of the results to a larger population difficult. These variations include sample characteristics, sun protection factor of LPA, dose regimen, a diagnostic protocol for RHL, and geographic differences. With regards to sample characteristics, the studies had different population characteristics in age, ethnicity, gender, and amount of outdoor activities. This introduces a source of heterogeneity in the pooled data, lowering the applicability of the results to a larger population. Additionally, there were variations in the strengths of SPF in the LPA used in these studies. For example, LPA used in some studies [30, 32] had an SPF of 15 while another study had an SPF of 30 [34]. One of the studies did not clarify whether the LPA used had any SPF, presenting a serious limitation to interpretation of its results [33]. Furthermore, variations in the dose regimen for applying LPA were found. Participants in Mills et al. applied LPA hourly while skiing, while those in Mazzarello et al. applied LPA every 2 hours [30, 34]. Variation in the geographic location of the studies also limited interstudy analysis. Shulman et al. conducted their study in a desert where a higher level of UVR is expected while Mills et al. was carried out at a ski resort [30, 33]. These interstudy variations introduce limitations in drawing a universally applicable finding. Moreover, the prevalence data obtained from the studies should be interpreted with caution. This is due to the possible confounding factors such as skin type, occupational exposure to sun, frequency of RHL, and application routine for LPA.
As suggested by all included studies, UV exposure is associated with RHL [30-34]. It is therefore important to identify the composition and application routine of LPA that can prevent RHL most effectively. Due to the limited data present and heterogeneity across the studies in the composition and application routine of LPA, it was not possible to identify the most ideal LPA. However, with UV filters being the critical ingredient in blocking UVR, it is arguable that LPAs containing higher SPF will be more effective. Further research can be done to find the most ideal composition and regimen of LPA.
Across the two observational studies and one RCT which explored the relationship between the use of LPAs and the presence of AC, collective data demonstrated that the use of sunscreen-containing LPAs was associated with a lower prevalence of AC lesions and remission of existing lesions [2, 21, 35]. All three studies for AC displayed an overall low risk of bias [2, 21, 35]. However, de Oliveira Ribeiro et al.’s observational study presented a major flaw in the inclusion of relevant data, by failing to specify whether sunscreen was applied to the lips specifically, reducing the quality of evidence significantly [2]. While de Oliveira Ribeiro et al. and dos Santos et al. determined the relationship between AC and LPA application, the cross-sectional nature of both the studies made it difficult to establish a causal relationship between factor exposure and disease development, as well as difficulty in distinguishing new presentations of the disease from those that have been present for some time [2]. De Oliveira Bezerra et al. also demonstrated that LPAs may be effective in the remission of some AC lesions [21]. However, this data may be more reliable if an additional group of subjects used a placebo lip agent, containing neither sunscreen nor fludroxycortide. Another limitation in the quality of evidence resulted due to missing data in de Oliveira Bezerra’s study. Although partial improvement was seen in a subject from the group which used fludroxycortide and LPA, adverse effects were reported [21]. Hence, the subject switched to using LPA only [21]. The outcome of this was not reported, implying biased results [21].
Overall, there was moderate quality of evidence that LPA use lowers the incidence of AC. Heterogeneity was evident across the three publications mainly in regard to study type and sample size. This was largely influenced by the study by de Oliveira Bezerra et al., which did not perform a sample size calculation and included a small sample size of 23 subjects, making the results inconclusive to a larger population [21]. Design limitations of de Oliveira Ribeiro et al. and dos Santos et al.’s studies contributed to the down-grading of evidence by one level, due to being observational in nature [2, 35].
It was difficult to ascertain the reliability of the prevalence rates obtained for AC in LPA users and LPA non-users due to confounding factors. In the studies conducted by dos Santos et al. and de Oliviera Ribeiro et al., length of sun exposure, other types of sun protection apart from LPS, and age were identified as confounding factors [2, 35]. Length of sun exposure varied across all participants which potentially affected AC prevalence. Type of sun protection is also considered a confounding factor. For example, wearing a wide-brimmed hat in addition to LPS would pose difficulty in determining AC prevalence in LPS users alone. Age is another confounding factor to be considered as it relates to the cumulative time of sun exposure over years for an individual, thus affecting the risk of AC development.
Across the studies, it was also important to determine an effective LPA application routine and SPF that would benefit in reducing the prevalence of AC. Dos Santos et al. and de Oliviera Ribeiro et al. did not study UV-filtering ingredients used by participants, and thus secondary outcomes could not be measured. Both studies did agree upon the fact that AC prevalence was greater with lack of LPA and greater exposure to UVR [2, 35]. In contrast, de Oliveria Bezerra et al. had participants in the control group who applied LPS only, with an SPF of 60, before sun exposure [21]. Less than half of the participants in this control group showed no clinical signs of improvements [21]. The majority of participants demonstrated improved AC symptoms which illustrated that LPS with an SPF of 60 applied before sun exposure can be effective in preventing AC [21]. However, it cannot be concluded that an LPA with SPF 60 is most effective in preventing AC in a wider population due to heterogeneity in results across the studies. Therefore, further studies are required to investigate an appropriate LPA in the prevention of AC.
It is widely accepted that AC is a potentially malignant disorder [7, 8, 35, 37-39]. As the malignant transformation of AC can have life-threatening consequences for the individual, prevention of AC must be considered a priority. Studies illustrate that sunscreen is a cost-effective preventative in reducing the risk of developing malignancy by protecting the skin against the harmful effects of UVR, which has been positively associated with the development of skin cancer [40-42]. Thus, as AC is a potentially premalignant condition, widespread awareness of AC prevention, particularly in prone communities, should be promoted.
One publication investigating the relationship between LPA and LC was included in our review. Pogoda et al. found that those living in a high UVR exposure region who applied LPA no more than once a day had twice the risk of developing LC (OR=7.3) compared to those who applied LPA more than once a day (OR=3.2) [36]. There is moderate quality evidence in this study supporting the use of LPA for the prevention of developing lip cancer. Despite having significant findings, this study contains some limitations. Due to the nature of the observational study design, participants were classified as cases with a confirmed diagnosis of LC between 1978 and 1985 or as controls, who were frequency matched by a decade of birth [36]. A verbatim questionnaire was then used in an interview to ascertain information on factors that may have increased the chances of developing LC, including the use of LPA, among those already diagnosed with LC and among controls [36]. Therefore, sufficient information regarding a specific intervention could not be ascertained from the study. Additionally, due to the nature of the study as cases were already diagnosed with LC, the results were presented as an Odds Ratio (OR) [36]. In this case, the exposure was the use of an LPA [36]. Risk of bias was introduced as the interviewer was not blinded to the LC status of the participants [36]. Furthermore, the study had a smaller sample size, reducing the quality of evidence in regard to precision. While the relationship between the use of an LPA and the presence of LC was explored, it was stated that most of the participants used coloured lipstick as the protectant [36]. Therefore, it is critical for future studies to investigate a specific LPA with an ideal level of SPF for the protection against LC.
Despite significant results, some general limitations in the studies were identified. Some studies had small sample sizes and restricted participant demographics, which decreased the quality of evidence with regards to precision [21, 31, 34]. Some studies had a lack of blinding of either participants and assessors which contributed to an increase in their risk of bias [33, 35]. One study also failed to mention whether the LPA used contained UV filters, which presented a serious limitation to the overall interpretation of the result [33]. Meta-analysis was not possible due to interstudy heterogeneity in the method, the type of LPA used, presentation of the results, and lack of clinical trials looking at the prevalence of the lip lesions.
Actinic cheilitis and lip cancer are lip lesions that can develop by prolonged exposure to UVR. Recurrent herpes labialis can also form by exposure to UVR as the dormant HSV-1 is reactivated. Our review of the current literature, with moderate quality of evidence suggests that sunscreen-containing lip protective agents can aid the prevention of actinic cheilitis and recurrent herpes labialis. However, there was limited evidence for the prevention of lip cancer with only one study being included for review, suggesting the lack of evidence in this area. The most effective formulation and application routine for lip protective agents could not be determined due to the limitations and heterogeneity across the studies and can be the focus of future studies. In addition, studies of non-English language or inaccessible full-text could not be included in this study. The results from our review may be applicable in clinical dentistry with respect to providing advice for the prevention of the lip-associated lesions to patients at high risk of UV exposure.
AUTHOR CONTRIBUTIONS
SM, AS, MR, AVS, and ST performed the systematic review and drafted the manuscript initially. AA and DS supervised the project and critically appraised and edited the manuscript.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGMENTS
The authors would like to deeply thank the JCU library staff for their support.


