Osseointegration of Hafnium when Compared to Titanium - A Structured Review
Abstract
Aim:
This systematic review was conducted to analyse osseointegration of hafnium over conventional titanium.
Materials and Methods:
Search methodology was comprehended using PICO analysis and a comprehensive search was initiated in PubMed Central, Medline, Cochrane, Ovid, Science Direct, Copernicus and Google Scholar databases to identify the related literature. Randomised control trials, clinical studies, case control studies and animal studies were searched for osseointegration of hafnium coated titanium implants versus conventional titanium implants. Timeline was set to include all the manuscripts published till December 2018 in this review.
Clinical Significance:
Hafnium is a very promising surface coating intervention that can augment osseointegration in titanium implants. If research could be widened, including in vivo studies on hafnium as a metal for coating over dental implants or as a dental implant material itself to enhance better osseointegration, it could explore possibilities of this metal in the rehabilitation of both intra and extra oral defects and in medically compromised patients with poor quality of bone.
Results:
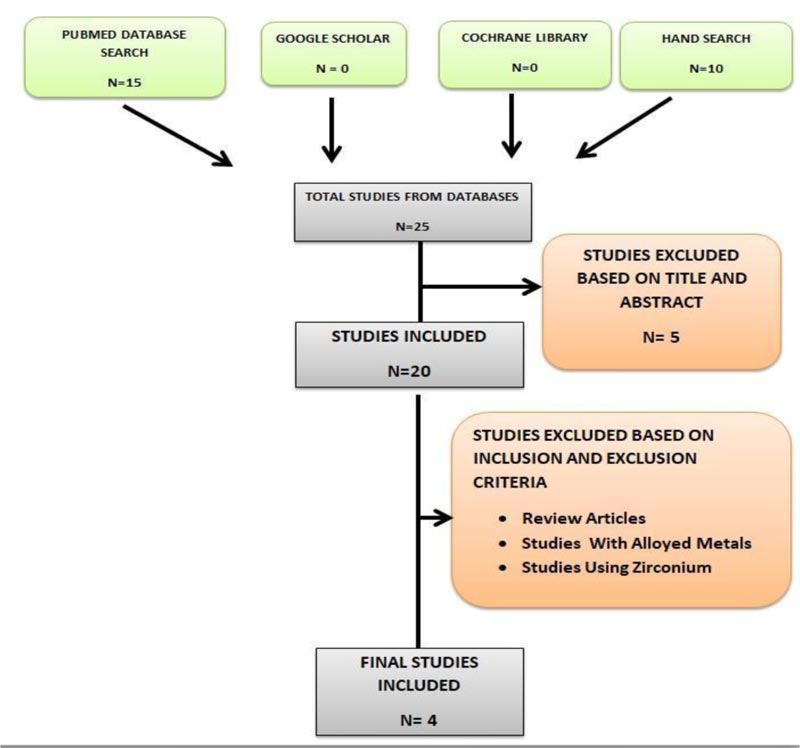
Out of the 25 articles obtained from the PICO based keyword search, 5 studies were excluded based on title and abstract. Out of the remaining 20 studies, 16 were excluded based on the inclusion and exclusion criteria of our interest and finally, 4 were included on the basis of core data.
Conclusion:
This systematic review observed hafnium metal exhibited superior osseointegration than titanium. Owing to its biocompatibility, hafnium could be an alternative to titanium, in the near future.
1. INTRODUCTION
The advent of tissue engineering provides a novel approach for the repair and reconstruction of bone defects [1-4]. An ideal implant material should have appropriate biocompa- tibility, corrosion resistance, elastic modulus, and favourable bone anchorage [5-12]. One of the most commonly used materials is titanium for its low elastic modulus, good corro- sion resistance and biocompatibility. Hence it has become the most commonly used biomaterial for dental implants [13-15].
In various studies conducted to date, Tantalum has revealed superior properties fulfilling the criteria required for an implant [16-20]. Tantalum has been shown to be a promising material for excellent chemical stability, fluid body resistance, biological inertness and remarkable osteocon- ductivity [16-26]. Tantalum has higher elastic modulus than human bone tissue but it’s prone to stress shielding effect [27]. To overcome this, porous forms of tantalum have been explored [28-30]. However, the structure of porous tantalum renders it unsuitable for long-term use in the load-bearing structures [31]. Hence tantalum porous implants with titanium substructures have become more popular [18, 31, 32]. Similarly, plasma spraying tantalum over titanium is also reported [33].
In the periodic table by IUPAC 2016, tantalum belongs to period 6 (d block) of the periodic table [34]. Hafnium belongs to the same block as tantalum, in the periodic table, hence similar biological and chemical behaviour analogous to tantalum are expected and therefore, hafnium coatings and their biological applications have been vigorously researched upon. The metal was first identified by Dirck Coster and Georges de Hevesy in Copenhagen in 1923 and owed its name to ’Hafnia’, the Latin name for Copenhagen. Hafnium is always found in association with zirconium in mineral ores [35-37]. The main mineral where it is found in zircon, with a ratio Hafnium/Zirconium of about 2.5% [38]. Hafnium is a passive metal with various properties, such as high ductility, strength, resistance to corrosion and mechanical damage. Due to a number of interesting properties such as high ductility and strength, as well as resistance to corrosion and mechanical damage, it has attracted interest for a number of applications [37]. For instance, it is used as a control material for nuclear reactors and as an alloying element in some superalloys used in aircrafts engines [39, 40].
Hafnium has also been investigated as an alloying element in titanium alloys. Different proportions of Titanium-Hafnium binary alloys have been studied and reported in the literature [41]. These alloys have shown a low elastic modulus which is beneficial in order to reduce the stress shielding effect and to enhance bone growth. It has also been shown that cold work can be used to decrease the elastic modulus of this type of alloy, reaching values close to the elastic modulus of cortical bone [42].
In 1984, Marcel Pourbaix proposed hafnium as a metal to be considered for surgical implants due to the passive state of the metal. However, due to the lack of information about its toxicity to the human body at that time, it was discarded from the final list of metals to be theoretically considered. More recently, the properties of hafnium as an implant material have been investigated. Studies have shown that hafnium metal had both good biocompatibility and osteogenic potential.To date, the literature illustrating the behaviour of hafnium as a surface coating in biological environments has been scarce. Thus, further studies of hafnium coating under biological conditions are needed in order to determine the suitability of this material, as a surface coating for biomedical applications. The aim of the current review is to systematically analyse the scientific evidence on osseointegration of hafnium coatings in titanium implants.
2. MATERIALS AND METHODS
2.1. Structured Question
Is osseointegration in hafnium significantly greater than titanium?
PICO [Problem, Intervention, Comparison, and Outcomes]
P- Osseointegration
I- Hafnium
C- Conventional titanium
O- Bone implant contact
2.2. Data Collection and Analysis
The studies selected were based on the data extraction and analysis of quality and publication bias. The data collection form was customized. The outcome measure was bone implant contact.
2.3. Literature Search Protocol
2.3.1. Sources Used
For identification of studies included or considered for this systematic review, detailed search strategies were developed for the database searched. The search methodology applied was a combination of MESH terms and suitable key words. The key words employed in this search were broadly classified into four categories describing population, intervention, outcome and the type of study. Key words within each group were combined using Boolean operator [OR] and the searches of individual groups were combined using Boolean operator [AND] to retrieve articles electronically. The protocol is registered in PROSPERO (acknowledgements of receipt (166932)).
2.3.2. Searched Databases
The electronic databases included were: PubMed, Google Scholar, Medline, Ovid, Science Direct, Copernicus, Cochrane database of systematic reviews and no limitation regarding publication type and the publication date was set.
2.3.3. Search Terms
P- osseointegration, Osteoblast cell adhesion, Fibroblast cell adhesion, Bone cement, Tissue adhesion, Cell adhesion, Cellular wettability, Bone bonding, Bone adhesion, Bone formation, Bone integration, Bone remodelling, Bone fusion, Bone implant junction, Bone regeneration
I- zirconium mineral, zirconium minerals, Zircon, Hafnium isotope, Hafnium isotopes, hafnium coating, Hafnium coatings, Hafnium surface coating, Hafnium surface coatings, Nanoparticle hafnium coating, Bio inert coating, Bio inert coatings, Hafnium compounds, Hafnium compound
C-Titanium implant, Titanium implant, Titanium alloy, Titanium alloys
O-Removal torque, Bone implant contact
2.3.4. Article Eligibility Criteria
The inclusion criteria include articles reporting bone regeneration with hafnium and healing with no restrictions on age or gender or ethnicity, studies on bone regeneration with titanium and its alloys, animal studies, in-vitro studies, RCT,case-series. The exclusion criteria include studies using zirconium containing hafnium, studies with metals other than pure hafnium and titanium, review articles, studies with metal coatings other than hafnium and titanium, studies with metal alloys other than titanium and hafnium.
2.3.5. Article Selection
The title and abstract of the entries yielded from the initial electronic database searches were read. After this initial filter, the full-text versions of the studies that could be potentially included in this review were read and a final selection of articles was made after applying the eligibility criteria.
2.3.6. Structured Algorithm
Search [bone bonding OR osseointegration OR Osteoblast cell adhesion OR Fibroblast cell adhesion OR Bone cement OR Tissue adhesion OR Cell adhesion OR Cellular wettability OR Bone implant contact OR Bone adhesion OR Bone formation OR Bone integration OR Bone remodelling OR Bone fusion OR Bone implant junction OR Bone regeneration] AND [titanium implant OR Titanium implants OR Titanium alloy OR Titanium alloys] AND [hafnium OR zirconium mineral OR zirconium minerals OR Zircon OR Hafnium isotope OR hafnium isotopes OR hafnium coating OR hafnium coatings OR Hafnium surface coating OR Hafnium surface coatings OR Nanoparticle hafnium coating OR Nanoparticle hafnium coatings OR Bioinert coating OR Bioinert coatings OR Hafnium compound OR Hafnium compounds] AND [bone implant contact OR Removal torque OR Resonance frequency analysis].
3. RESULTS
Out of the 25 articles obtained from searching all databases, 5 studies were excluded based on title and abstract. Out of the remaining 20 studies, 16 were excluded based on the inclusion and exclusion criteria of our interest and 4 were included on the basis of core data (Table 1). The 4 articles were reviewed and were consolidated as depicted in the flowchart below (Fig. 1).
The treatment effects measured in these studies were bone-implant contact, percentage of new bone formation, cellular adhesion, and osteoblastic activity (Table 2).
The data of the selected studies were extracted using standardized abstraction tables. Information extracted from each study included the following in one table as general characteristics of the study: 1) Title 2) Author and year 3) Study design 4) Duration 5) Intervention 6) Groups 7) Sample size 8) Types of statistical methods used 9) Outcome measures Table 3.The outcome variables of the extracted data from the studies were interpreted in detail (Table 4). The level of evidence, according to Oxford Centre for Evidence-Based Medicine 2011, was also tabulated (Table 5).
4. DISCUSSION
This Systematic review reveals four articles evaluating osseointegration of hafnium over the gold standard metal titanium [43-45]. The studies show evidence that hafnium appears to have equivalent biocompatible properties as compared to Tantalum, Rhenium and other implant materials. However, the exclusions were not statistically significant and so larger studies with the stronger design are required to provide conclusive evidence on the exact effectiveness of Hafnium on osseointegration in human osseous tissues. A Meta-analysis could not be performed with the studies included, as the outcome parameters measuring the osseointegration were different in all the studies.
The studies included in this review show significant bone gain with hafnium implants. All four included studies evaluated different outcome parameters making it difficult to consolidate the results over a single outcome measure. The outcome parameters used to study osseointegration in the studies included in this review were bone-implant contact, percentage of new bone formation, alkaline phosphatase levels in blood, cellular adhesion and cellular proliferation [26, 46-48].
| AUTHOR & YEAR | STUDY DESIGN | REASON FOR EXCLUSION |
|---|---|---|
| Akhtiamov et al. 2015 | Animal study | Difference in the intervention group and outcome parameters |
| Herranz-Diez, et al. 2016 | In-vitro study | Difference in intervention and outcome parameters |
| Jeong et al. 2009 | In-vitro study | Difference in intervention group |
| Akhtiamov et al. 2015 | Animal study | Difference in the intervention group |
| Wang et al. 2014 | Literature review | Review article |
| Herranz-Diez et al. 2015 | In-vitro study | Difference in intervention group and outcome parameters |
| Liu et al. 2017 | Literature review | Review article |
| Sin et al. 2013 | In-vitro study | Difference in outcome parameters |
| Qin et al. 2018 | Literature review | Review article |
| Benic et al. 2017 | Animal study | Intervention Group Contains Different Metal |
| Wang et al. 2016 | Animal study | Difference in intervention group |
| AlFarraj AA et al. 2018 | Animal study | Difference in intervention group |
| Cho Y et al. 2015 | In-vitro study | Intervention Group Contains Different Metal |
| Kang HK et al. 2013 | Animal study | Intervention Group And Comparison Group Contains Different Metal |
| Diefenbeck M et al. 2011 | Animal study | Different Problem parameter |
| Shin D et al. 2011 | Animal study | Intervention Group Contains Different Metal |
| Wen B et al. 2016 | Animal study | Difference in intervention group |
| Wenz et al. 2008 | Systematic Review | Review article |
| Kong YM et al. 2002 | Animal study | Difference in intervention group |
| Dubruille JH et al. 1999 | Animal study | Difference in intervention group |
| Li J et al. | Animal study | Difference in intervention group |
| TYPES OF OUTCOME MEASURES |
|---|
| Bone Implant Contact |
| New Bone Formation |
| Alkaline Phosphatase Levels |
| Cellular Adhesion And Osteoblastic Activity |
| TITLE | AUTHOR YEAR | STUDY DESIGN | TIME PERIOD | INTERVENTION | GROUPS | SAMPLE SIZE |
STATISTICS | OUTCOME MEASURES |
|---|---|---|---|---|---|---|---|---|
| Tissue response to hafnium | Mohommadi S et al. 2001 |
Animal study | 24 WEEKS | machined Hafnium non-threaded implants | Group 1=Hafnium implants in abdominal wall Group 2=Titanium implants in abdominal wall Group 3=Hafnium implants in Tibia Group 4=Titanium implants in tibia |
N= 78 Group 1= 21 Group 2= 21 Group 3=18 Group 4= 18 |
Fishchers test, T test | 1]tissue-implant interface were evaluated by light microscopy [morphometry] 2]Bone-implant contact and bone area within threads were evaluated in ground sections |
| Biocompatibility & osteogenesis of refractory metal implants, titanium, hafnium, niobium, tantalum & rhenium | H. Matsuno et al.. 2001 | Animal study | 4 WEEKS | refractory metal implants |
titanium, hafnium, niobium, tantalum and rhenium wires | Not mentioned | one-factor ANOVA, Fisher's & Kruskal Wallis test. |
Surface structure and roughness SOFT TISSUE: optical microscopy, X-ray scanning analytical Microscope & HARD TISSUE:optical microscopy, electron probe microanalyzer, reflected electrons, new bone formation |
| Effect of hafnium and titanium coated implants on several blood biochemical markers after osteosynthesis in rabbits | Yousef et al. 2014 | Animal study | 60 days | Medical steel 12Х18H9T [C-0.2%; Si0.8%; Mn-2%; Ni-[8-9.5]%; S-0.02%; P-0.035%; Cr [17-19]%; Cu-0.3%; Fe-67%], coated with titanium and hafnium nitrides |
Test group=medical steel coated with titanium and hafnium nitrides, with a diameter of 2 mm control group =non-coated medical steel with the same diameter was used |
N =30 Individual group sample not mentioned |
Student’s t-test with a Bonferroni correction |
1]alkaline phosphatase [ALP] [kinetic colorimetric method using ALP DGKC system test 2]level of calcium [photometric method] 3]phosphorus [spectrometric method 4]total protein, aspartate aminotransferase and alanine aminotransferase [AST, ALT], 5]the level of glucose [test system GLUC-PAP] |
| Cellular responses of osteoblast-like cells to 17 elemental metals | Zhang et al. 2016 |
In vitro study. | 168 hours | Pure elemental metals | titanium[Ti], zirconium[Zr], hafnium[Hf], vanadium[V], niobium[Nb], tantalum[Ta], Chromium[Cr], molybdenum[Mo], manganese[Mn], iron[Fe], Ruthenium[Ru], cobalt[Co], nickel[Ni], copper[Cu], zinc [Zn], silicon[Si] & tin[Sn] | N=17 | One-way ANOVA with post-hoc Turkey HSD | 1]Protein adsorption 2]Cell adhesion 3]Cell proliferation 4]Cell morphology and actin cytoskeleton 5]Ion release 6]ALP activity and collagen content |
| AUTHOR YEAR |
||||||
|---|---|---|---|---|---|---|
| OUTCOME MEASURE | MEAN ± SD | NUMBER OF CELLS |
PERCENTAGE OF NEW BONE FORMATION | P VALUE | CONCLUSION | |
| Mohommadi et al. 2001 | Bone-implant contact | - | - | - | P>0.05 | Hafnium and titanium were similar in inducing osteogenic properties. |
| H. Matsuno et al.. 2001 | percentage of new bone formation | - - |
- | After 2 weeks:10% for all the implants After 4 weeks: percentage had markedly increased for each metal |
After 2 weeks p>0.05 After 4 weeks P<0.05 |
The results of animal implantation test of Titanium, Hafnium, Niobium, Tantalum and Rhenium in both soft and hard tissue of rats showed that they have good biocompatibility and osteogenesis. |
| Yousef et al. 2014 | Alkaline phosphatase | 5th day post-operative Test[coated]=166.16±18.56 Control[uncoated]=146.36±18.63 60th day post-operative Test[coated] =136.27±15.87 Control[uncoated]=142.41±21.62 |
- | - | 5th day P<0.05 60th day p>0.05 |
Nano-technologically coated implants with a bio inert combination of titanium and hafnium nitrides for the purpose of prevention of the possible complication, such as individual intolerance of patient to the implants. There was no difference between the groups after 60 days. |
| Zhang et al. 2016 |
Cellular adhesion &cellular proliferation | - | No. of cells adhered on Ti & Hf discs increased gradually upto 4h & no. of SaOS2 cells significantly higher than control group after 168h | - | P<0.05 | Good cell proliferation was observed on discs of group 1 metals comprising Titanium, Hafnium etc. |
| STUDY | STUDY DESIGN | CEBM LEVEL OF EVIDENCE |
|---|---|---|
| Mohommadi S et al. 2001 | Animal study | Level 5 |
| H. Matsuno et al.. 2001 | Animal study | Level 5 |
| Yousef et al. 2014 | Animal study | Level 5 |
| Zhang et al. 2016 | In vitro study. | Level 5 |
It is well established that measuring bone implant contact is the standard gold technique for the measurement of osseointegration in animal models [49-51]. Similarly, measuring the cell proliferation of osteoblastic cell lines is the gold standard technique for in vitro studies [52-54]. Hence it is justifiable to give more weightage to the studies measuring the gold standard outcome measures [55-59]. Apart from the above-mentioned parameters, the biochemical marker alkaline phosphatase is also considered an adjunct aid to prove significant osseointegration [52, 60, 61].The current evidence in the available literature shows that hafnium also promotes superior osteogenic cell proliferation when compared to titanium. The limitations of this review are the in vitro nature of the studies included with level 5 evidence, in vivo intervention in animal models and the absence of randomized control human trials with both titanium and hafnium coatings over the implant surfaces in varying clinical situations [58, 62]. Hence the inference needs to be interpreted prudently [63-67].
CONCLUSION
Based on this systematic review, hafnium is a very promising surface coating intervention that can augment osseointegration in titanium implants. However, this needs to be validated through rigorous long-term clinical trials. Owing to its biocompatibility and osseointegrative properties, hafnium could be an alternative to titanium, in the near future.
CLINICAL SIGNIFICANCE
Hafnium is a very promising surface coating intervention that can augment osseointegration in titanium implants. If research could be widened including in vivo studies on hafnium as a metal for coating over dental implants or as a dental implant material itself to enhance better osseointegration, it could explore possibilities of this metal in rehabilitation of both intra and extra oral defects and in medically compromised patients with poor quality of bone.
CONSENT FOR PUBLICATION
Not applicable.
STANDARDS FOR REPORTING
PRISMA guidelines and methodology were followed.
FUNDING
None.
CONFLICT OF INTEREST
The author declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We thank all the authors for their contribution to this research paper.


