All published articles of this journal are available on ScienceDirect.
Actinomyces Infection after Permanent Fillers Lip Augmentation: Diagnosis and Treatment
Abstract
Background:
Although dermal filler injections are a widespread and secure practice, early or late complications may nonetheless occur.
Objective:
In this paper, the authors report an unusual asymptomatic late filler infection caused by Actinomycetes in a patient having received liquid silicone and acrylate filler injections for lip enhancement, seeking treatment for upper lip macrocheilia.
Methods:
The case report is about a patient who complained of recurrent lip region edema and denied any infective episodes. Preoperative analysis was in the normal range. Sonographic exam showed two fillers in the upper lip, suggestive of silicone and polyacrylamide gel. A conservative macrocheilia reduction was performed; healing was uneventful.
Results:
Histology report confirmed the preoperative sonographic diagnosis, together with an actinomycetes infection, clinically unsuspected. Many microorganisms have been reported to cause abscesses or granuloma after dermal filler treatments, sterile abscesses were reported as well, suggesting a biofilm infection as causative granuloma origin.
Conclusion:
This study differs from previous filler complication reports because of the occasional finding of a silent actinomyces infection, a commensal of the oral cavity but responsible for suppurative diseases in the soft tissues. This occasional finding seems to support the biofilm origin of dermal filler granuloma.
1. INTRODUCTION
In recent years many patients seek cosmetic treatments to reduce the effects of the photoaging and chronoaging process. Dermal filler is the golden standard to fill up facial rhytids, with more than 3.700.000 treatments performed worldwide in 2018 [1] and a continuous rising in the number of treatments performed each year. Injectables may be of temporary (Hyaluronic acid, collagen, etc.) or long lasting and permanent (calcium hydroxyl apatite, poly-L-lactic acid, polymethylmethacrylate, silicon, etc.) nature [2], according with their permanence in the tissues, variable from few months to an everlasting stay.
Although dermal filler injections are a safe procedure, early or late complications may still occur [3]. Early complications are more often bruising, infections, allergic reactions, while late complications are nodule formation, recurrent infections, chronic oedema [4-6]. In this paper, the authors report an unusual asymptomatic late filler infection caused by Actinomycetes in a patient who received liquid silicone and acrylate filler injections for lip enhancement.
2. CASE REPORT
Sixty-year-old female patient presented to the first author’s private setting, seeking treatment for recurrent lip region edema, treated in the past with general corticosteroid therapy and antibiotics with transient effects only.
Patient reported upper lip filler injection performed in a medical setting twelve years ago for cosmetic purposes. A “touch up” upper lip injection was performed by the same doctor two years after the first treatment. Patient ignored which dermal filler was injected in both sessions.
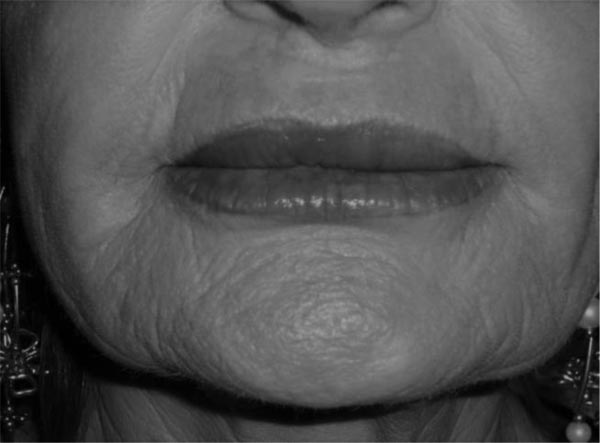
The patient majorly complained about chronic, painless upper lip swelling, vermilion distortion, lip hardening with rubbery consistency at palpation (Fig. 1). There was no active inflammation and the patient denied any purulent discharge episode.
High-resolution sonography was performed a Hitachi H21 apparatus (Hitachi Medical Corporation, Tokyo, Japan) equipped with a high-resolution probe (10-13 MHz for small parts) according to a known protocol [7-9]. The image was consistent for diffuse lip infiltration with silicone, along with few spots of another acrylate filler (Fig. 2).
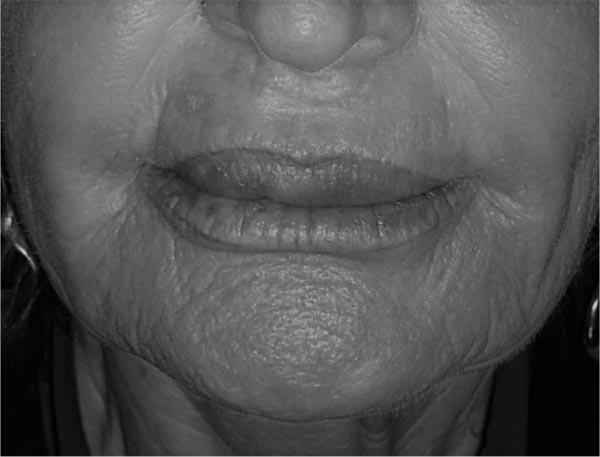
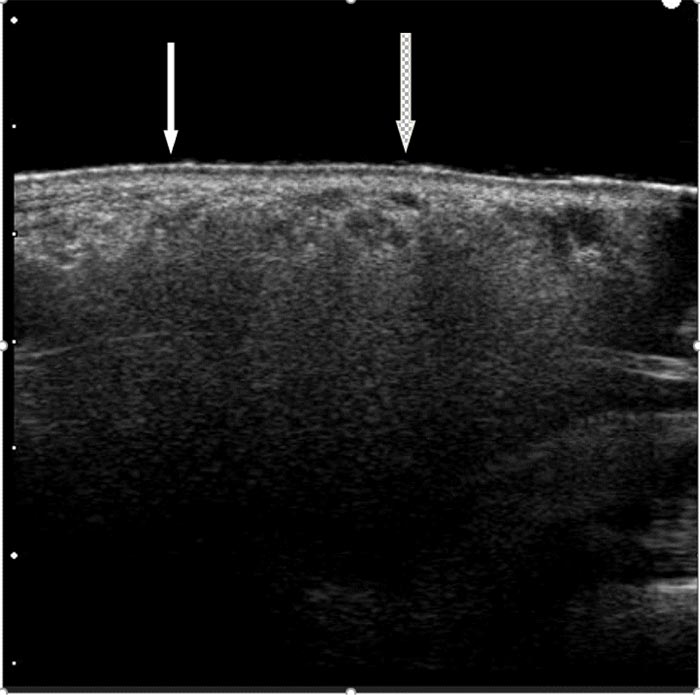
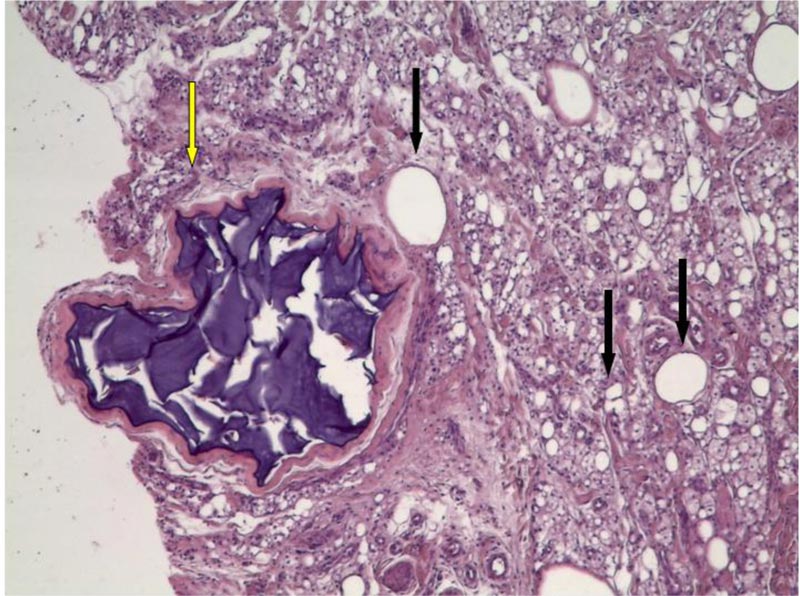
A reduction conservative cheiloplasty procedure was proposed to this patient to improve the upper lip macrocheilia aiming to restore a smaller lip volume while removing some of the permanent fillers’ component.
Preoperative analysis (full blood count, CRP and VES exams) were all in the normal range.
Surgery was performed in outpatient regime, with regional block anesthesia, with no soft tissue distortion. A conservative elliptical vermilion excision aiming to reduce part of the bulkiness of the upper lip and to restore lip symmetry was performed, according to a known procedure [10]. Tissues removed during surgery were embedded in 4% buffered formalin and sent to the pathologist.
Histology report confirmed the preoperative sonographic diagnosis of upper lip augmentation with silicone and polyacrylamide gel (PAAG).
Histology report showed the thickness of dermis, a marked inflammatory reaction almost exclusively composed with histiocytes engulfed with foreign body and with bubbled and foamy appearance of cytoplasm, confirming the clinical and sonographic diagnosis of siliconoma. A second filler was detected within a foreign body granuloma, as for PAAG. (Fig. 3)
In the focused area, a small granuloma was present encircled by a rim of histiocytes with small nuclei and scant cytoplasm arranged around a fibrillary basophil core of filiform bacteria, resembling a small mycetoma. Neutrophils were not evident. The morphology of the sample was consistent with silicone reaction with actinomycetes infection, clinically unsuspected (Fig. 4) (see figure, Supplemental Digital Content 1, which demonstrates actinomycetes filaments at H&E stains x 400).
Patient received Clindamycin 600 mg daily for three weeks. Healing was uneventful.
3. FOLLOW UP
The patient returned to the outpatient clinic for follow-up visits at six months intervals, with a follow-up of five years, with no upper lip inflammation episodes or enlargements (Fig. 5).
4. DISCUSSION
Many patients, as in this present case, ignore the product injected into their tissues. Radiologic exams can be of help in detecting a single product or, at least, a product family (temporary vs permanent) [7, 8, 11].
Numerous authors reported on the histological pattern of diverse filler, giving a pathway to identify the injected product even in absence of the product identification label [12-14]: according to these morphologic criteria the presence of both Silicone and PAAG in this patient’s specimen were established.
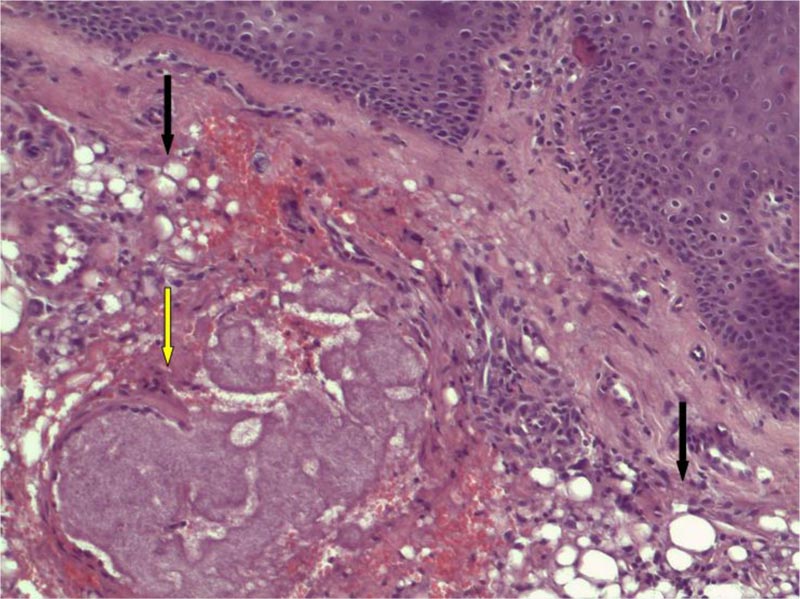
Many microorganisms have been reported to cause abscesses or granuloma after dermal filler treatments [15, 16], with more often involved common skin and soft tissue pathogens such as S. aureus or Klebsiella.
Sterile abscesses have been frequently reported, as well as biofilm low grade infection, in causing granuloma, erythema, swelling or pain [17].
This study differs from previous filler complication reports because of the occasional finding of a silent actinomyces infection within the upper lip tissues, revealed only by histology.
Actinomyces are non-spore forming, anaerobic gram-positive bacteria, commonly found in the oropharynx as non pathogen commensal [18], and in oral biofilm.
When actinomyces disrupt the mucous barrier, they become causative agent of a rare chronic suppurative infection characterized by soft tissues or lung or abdominal abscesses with multiple sinus formation [19].
Microbial biofilms develop when microorganisms adhere to a surface and produce extracellular polymers that facilitate adhesion and provide a structural matrix [20]. Biofilm causes a low-grade infection characterized by low host response, high antibiotic resistance, and their presence is difficult to detect in microbiologic culture. Biofilm can become activated because of bacteremia or trauma, and once active, they can cause acute purulent infections and sepsis or chronic inflammation with a subsequent granulomatous response.
Christensen et al. reported the better sensitivity of epi-fluorescence microscopy with peptide nucleic acid probes (PNA FISH) when compared to traditional histology, in detecting cocci in cluster surrounded by a smear of extracellular DNA which supposed be part of a biofilm matrix in patients with nodule, tissue swelling and pain after filler injection [17].
Wang proposed biofilm colonization in acrylates or silicone filler as promoting agents for granuloma formation [21], and Actinomyces has been proved to cause biofilm production on intrauterine copper devices [22], but this microorganism was never associated with biofilm involved in dermal filler complications.
CONCLUSION
In this patient, it is virtually impossible to ascertain the time and modalities of the actinomyces contamination. Actinomyces colony were observed secluded within a granuloma, not in direct contact with soft tissues, in absence of superinfections with other pathogens that could have triggered an immune reaction with purulent discharge or other pathological clinical signs, as confirmed by the lack of granulocytes in the histological specimen and the preoperative blood tests in the normal range.
The presence of this actinomyces colony could indirectly confirm the biofilm hypothesis on granuloma origin, as seen in the PAAG granuloma and silicon granuloma present in the specimen.
A cultural swab to confirm the actinomyces infection was not performed intraoperatively because there were no signs of infection nor purulent material, to send to the microbiology laboratory.
Prolonged Clindamycin therapy, whose efficacy against oral actinomyces is known [23], in association with removing part of the foreign material from lip tissues, has proved to be an efficient strategy in long term result, with no inflammatory episodes in this patient and a good aesthetic correction of the upper lip macrocheilia.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The authors confirm that the study was for the diagnostic records and the treatment was administered to the patient according to normal procedures. Therefore, no ethical approval was required.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
The patient gave her written consent for the execution of the diagnostic and therapeutic procedures and for the publication of the case report.
STANDARDS OF REPORTING
CARE guidelines and methodology were followed.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


