All published articles of this journal are available on ScienceDirect.
Cone Morse Implant Placement in Patients With Aids Who Use Highly Active Antiretroviral Therapy Report of Clinical Cases
Abstract
Introduction:
The Acquired Immune Deficiency Syndrome (AIDS) is a condition that manifests itself after the infection of the human organism by the Human Immunodeficiency Virus (HIV). In 1996, the Highly Active Antiretroviral Therapy (HAART) was introduced, with the aim of slowing down the immunodeficiency and restoring the immunity of these patients, extending their life expectancy. Consequently, the need for rehabilitating dental treatments arose, aiming to improve oral health, self-esteem and the quality of life of these patients. This current study was designed to assess vertical dimensional changes in the peri-implant bone level around the placement of dental implants in AIDS patients using HAART.
Materials and Methods:
For the bone level evaluation, at first cone-beam computed tomography, panoramic radiography and periapical radiographs were used during the periods at baseline, 2, 4 and 6 months after the implant installation. The images were digitized and analyzed on programs Adobe Photoshop CS5 and Digimizer 3.1.1.0.
Results:
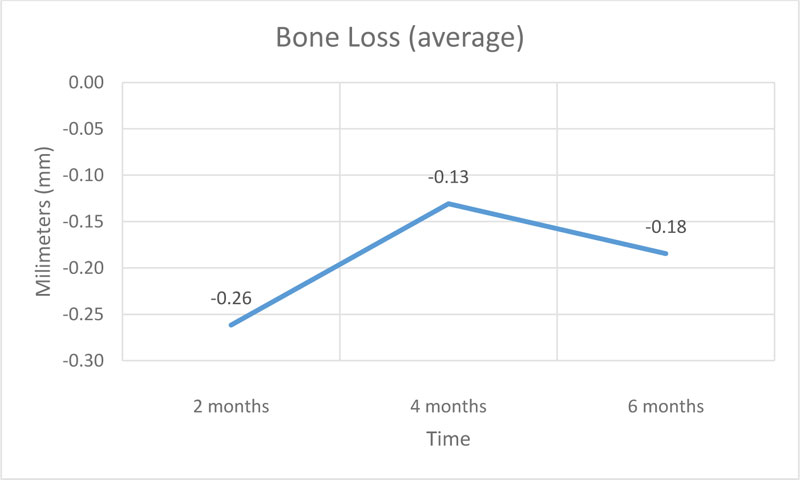
Were installed 13 implants that presented a peri-implant bone loss average of 0.26 mm in the first bimester, 0.13 mm in the second and 0.18 mm in the third, totalizing a peri-implant bone loss average of 0.57 mm in the semester.
Conclusion:
Despite the several metabolic changes that can affect these patients due to infection, drug therapy, immune response and the absence of an adequate stability quotient and insertion torque, all implants showed osseointegration, as well as the parameters of clinical success after the installation of the implant, and the degree of bone loss in this period is within the expected according to the research.
Clinical Relevance:
Oral health professionals should be aware of the possible complications that future HIV patients may have due to their systemic and drug-related condition in association with osseointegration.
1. INTRODUCTION
The Acquired Immune Deficiency Syndrome (AIDS) is a condition that manifests itself after the human organism infection by the Human Immunodeficiency Virus (HIV). According to UNAIDS, in 2018, approximately 37.9 million people were living with HIV and there were 1,7 million new infections in the world, in Brazil, this number is around 610 thousand to 1 million people living with HIV and approxi-mately 31 to 57 thousand new HIV infections per year [1].
The HIV infection leads to a chronic and usually fatal infection that is characterized by progressive immuno-deficiency, with an extended period of clinical latency, and opportunistic infections. The condition is categorized by infection and replication of HIV between T-lymphocytes expressing the CD4 antigen (helper inducer lymphocyte) [2]. In 1996, there was a new hope with the introduction of Highly Active Antiretroviral Therapy (HAART), since it was especially projected to the specific inhibition of viral replication through antiretroviral drugs, the main objective of which is to delay the immunodeficiency progression and/or to restore the immunity, extending the lifetime and quality of life of the infected person. However, nowadays, we have noticed a high occurrence of adverse side effects and inherent resistance to the therapy itself [2-6].
This is the new reality and concern faced by these professionals, since the HIV infection can be controlled, the viral load can be suppressed, the immunity can be stabilized, resulting in a higher survival rate, due to the use of an effective and lasting antiretroviral therapy. After HAART started being used, scientific findings showed that bone metabolism could be closely linked to the use of these products. The gradual bone demineralization is the characteristic of individuals infected with HIV that are treated with protease inhibitors, whereas these showed a higher chance of having a more significant bone demineralization, that can be associated with an increasing prevalence of osteopenia and osteoporosis, that can increase the risk of bone fractures in people with HIV/AIDS [7-14].
Individuals with Aids that are administrating HAART, showed a higher propension to have a more significant bone demineralization, that can be associated with an increasing prevalence of osteopenia and osteoporosis, increasing the risk of bone fractures and/or making the peri-implant bone formation more difficult. Therefore, the purpose of this research is to evaluate the bone level and the clinical success of the implants installed in patients with Aids who use HAART.
2. MATERIALS AND METHODS
The research project was approved by the Research Ethics Committee of Paulista University (UNIP), under opinion number 1322411. There were selected patients who seek care at the Center for Study and Care of Special Patients (CEAPE) and Implantology Course/Credit of the Postgraduation Program and Research of UNIP (FOUNIP). The research was conducted at the CEAPE of Paulista University Dentistry Faculty (Fig. 1).
The patient should be in AIDS and administrating highly active antiretroviral therapy (HAART), not having at least one tooth and present satisfactory oral and general health. All patients underwent a complete clinical examination, including anamnesis, physical examination and laboratory tests. Before the surgical procedures, the patients were molded for the case planning and surgical guides were made, which were used in the implant placement surgery.
Before installing the implants, the following procedures were performed: extra-oral antisepsis of the patient with chlorhexidine 2%, intra-oral antisepsis with chlorhexidine 0.12% (rinsing the mouth for 1 minute), surgical field assembly and sterile field placement, local anesthesia (lidocaine 2% with vasoconstrictor), linear incision over the border and mucoperiosteal flap detachment. The bone perforation for the implant installation was conducted according to the manufacture's instruction.
The patients were medicated with amoxicillin 500mg: 1 tablet every 8 hours during 7 days, with the first double dose being administered 1 hour before the surgery; dexamethasone 4mg: 1 tablet every 24 hours during 3 days, with the first dose being administered 1 hour before the surgery; and paracetamol 750mg: 1 tablet every 8 hours during 3 days, in case of pain (Fig. 2).
The patients who underwent implant placement surgery were instructed to avoid mechanical cleaning in the operated region. To clean the region, mouthwashes with 0.12% chlorhexidine were instituted twice daily, for 7 days. Nylon sutures were removed 10 days after surgery.
After 6 months, the procedures of prosthetic components selection, components installation and molding for the manufacture of screwed metal-ceramic crowns were perfomed.
2.1. Evaluated Parameters
2.1.1. Bone Loss
The radiographic control was performed by periapical radiographs taken immediately after implant placement and during the following periods after the surgery: 2, 4 and 6 months. As level bone evaluation reference was used, the peri-implant bone of the mesial and distal sides were found in periapical radiographs (Figs. 3, 4).
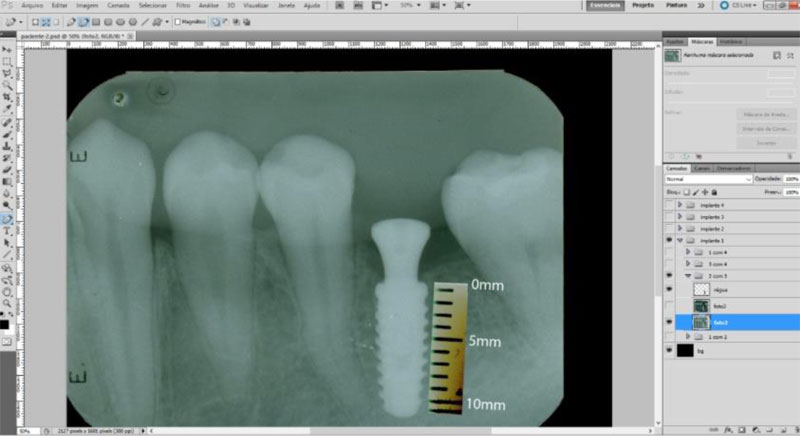
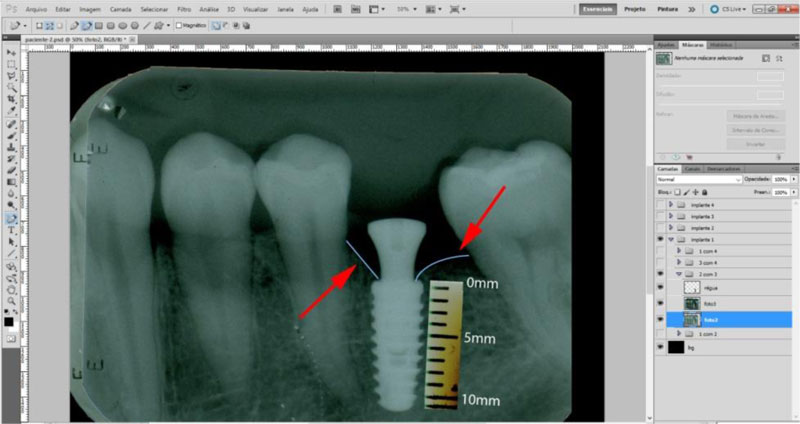
The periapical radiographs were obtained using the parallelism technique by an individualization (bite guide) of the radiographic positioning (Cone Est. Química - Maquira), using a condensation silicone (Optosil- Comfort Putty) at the teeth support site, making the patient biting always in the same position. Radiographic film (Contrast Speed E-DFL) was used with SpectroII X-ray equipment (Dabi-Atlante, Brazil), with a peak kilovoltage fixed at 67, an exposure time of 0.6 seconds and fixed milliamps at 8mA.
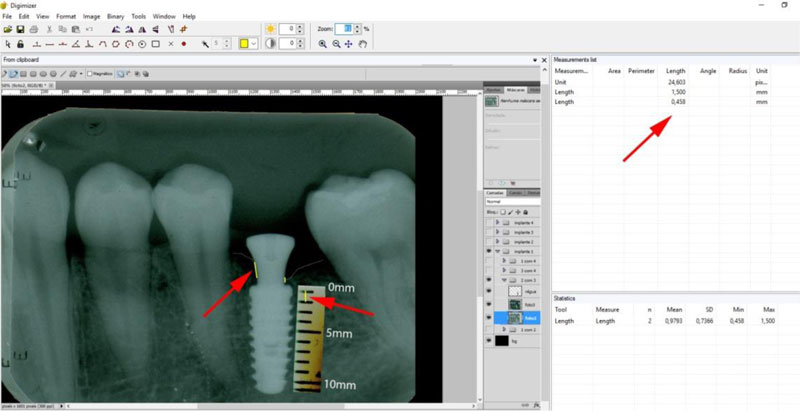
The periapical radiographs images, generated in the chemical radiographic processing, were made using the temperature-time method. These images were obtained and transferred to a computer with a Scanner HP-Scanjet G4050, in order to analyse them using the programs Adobe PhotoshopCS5 and Digimizer version 3.1.1.0, getting linear measurements of vertical peri-implant marginal bone levels converting pixels to millimetres. After 6 months, the implants must present clinical patterns of success, such as the absence of pain, infection, mobility and continuous radiolucency around the implant (Fig. 5).
3. RESULTS
The patients were screened at CEAPE FOUNIP, resulting in a total of 13 installed implants.
During the evaluations on the periods of 2, 4 and 6 months, the patients reported the absence of pain and the absence of infection, mobility and continuous radiolucency around the implant were clinically observed. No implant was lost during the evaluation period.
4. DISCUSSION
After the introduction of HAART, in 1996, the AIDS development parameters, which occur after the human organism infection by HIV, experienced a significant change. HAART has the purpose of slowing down the immunodeficiency and restoring immunity. Thereby, patients who use this therapy have a higher survival rate and better quality of life, creating, then, the need for rehabilitative treatments for this group of patients. Despite several positive aspects, this therapy has side effects that can be enhanced by infection, such as changes in bone metabolism [2-6].
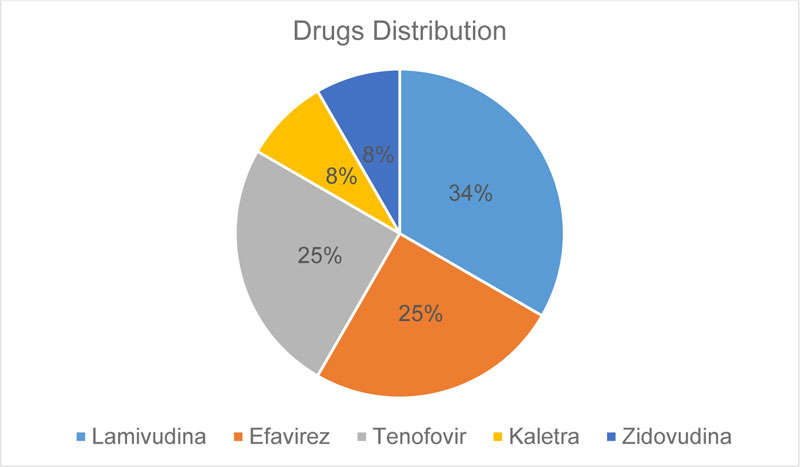
This study had a sample of 13 implants, with an average age of 45 years. All patients use Lamivudine (nucleoside analogue reverse transcriptase inhibitors), 25%of patients use Efavirenz (non-nucleoside analogue reverse transcriptase inhibitors), 25% of patients use Tenofovir (nucleotide analogue reverse transcriptase inhibitors), 8% of patients use Zidovudina and Kaletra (protease inhibitors). These drugs can cause several clinical changes, such as metabolic disorders, cardiovascular diseases, changes in liver and kidney functions, decreased cognition and changes in bone metabolism, like osteopenia and osteoporosis [15-18]. However, all patients were within normal limits, determined by laboratory tests.
According to the literature, HAART may be associated with an increased prevalence of osteopenia and osteoporosis, which can raise the risk of bone fractures in people with HIV/AIDS [7-14], as well as altering the bone/implant osseointegration relationship, promoting the development of peri-implantitis and/or loss of the implant. Therefore, this study assessed the peri-implant bone level using periapical radiographs, in addition to clinical evaluation. According to the criteria established by the European Commission (2012), computed tomography after implant installation should only be performed in cases where it is necessary to assess complications such as nerve damage and infections. For routine exams, the most suitable protocol is periapical radiography [19].
In this research, cone morse connection implants were used, that, according to researchers [20-22], have a reduced platform that minimizes the bone loss around the implants, that can occur by reducing bacterial infiltration and bone crest tension due to a better distribution of masticatory strength, which is very important since these patients are more likely to have bone resorption.
The results obtained at baseline and 2 months demonstrate an average bone loss of 0.26 mm, in the period of 2 and 4 months, the average bone loss was 0.13 mm and in the period of 4 and 6 months, the average bone loss was 1.18 mm. The average total bone loss was 0.57 mm, which supports established researches that showed a 1.5 to 2.0 mm peri-implant loss after the first year of installation in normoreactive patients [23-25].
There are not many studies related to the installation of implants in patients with HIV, but despite this, it was possible to find two studies. A study carried out in 2012 reported that the average bone loss in a group of patients who were using HAART with the protease inhibitor was 0.49 mm, and in the group who was using HAART without the protease inhibitor, the average bone loss was 0.47 mm, within one year [26], indicating that our study shows a higher bone level loss.
However, another study published in 2016 showed that the average peri-implant bone loss in patients with HIV was 1.19 mm in one year [27], which shows greater bone loss than our study, but this could have happened due to the shorter follow-up time of the study. It is worth mentioning that, in this study, the highest level of bone loss, when compared to the number of CD4 T cells, proved to be inversely proportional, that is, the greater the number of cells, the lower the level of loss.
Currently, the appropriate parameters to measure implant stability are the implant stability quotient and the insertion torque [28, 29]. Typically, the implant stability quotient scale ranges from 40 to 80 [30]. Due to the bone quality found at the time of surgery, all 13 implants reached implant stability quotient <40 and insertion torque <20 N/cm. Despite this, during the first 6 months after the surgery, all implants were osseointegrated.
The cause of periodontitis is an integral part of a multifactorial condition. The presence of subgingival biofilm, together with a different immune response from the host, may be essential for the development of this disease [31, 32]. Due to the combination of factors and mainly to the possible different immune response from the hosts to an inflammatory process, which can potentiate and accelerate that situation, the motivation of the patient and in conjunction with the procedures performed by the oral health team, it is extremely important to control the bacterial plaque, which is a factor that can be controlled and, consequently, can improve the inflammatory response of individuals who have metabolic and immunological changes.
In addition, the clinical parameters of the absence of pain, infection, mobility and continuous radiolucency around the implant were noticed in the clinical examination of patients, confirming the criteria established since the 1980s [26, 33].
CONCLUSION
Despite the various metabolic changes that can affect these patients due to infection, drug therapy, immune response and the absence of an adequate stability quotient and insertion torque, all implants showed osseointegration, as well as the parameters of clinical success after implant installation, and the level of bone loss in this period is within the expected according to the research. This research needs to have a longer follow-up time, as well as a larger number of patients to validate the purpose of this study.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The research project was approved by the Research Ethics Committee of Paulista University (UNIP), Brazil under opinion number 1322411.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Not applicable.
STANDARD OF REPORTING
CARE guidelines and methodology were followed.
FUNDING
None.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


