All published articles of this journal are available on ScienceDirect.
Effect of Fluoride Gels on the Surface Roughness of Different Composite Resins
Abstract
Background:
The pH of fluoride gels influences the roughness of composite resins, which affects their clinical durability.
Objective:
To evaluate the effect of fluoride gels (1.23% acidified phosphate fluoride and neutral fluoride) on the morphology of different composite resins after finishing and polishing.
Methods:
We prepared sixty specimens with a diameter of 5 mm and a height of 2 mm. The specimens were then divided into six groups (n = 10), according to the type of composite resin (Z250 micro-hybrid (Z), Filtek Z350 XT nanoparticles (XT), and Tetric N-Ceram nano-hybrid (TC)) and fluoride gel treatment (1.23% acidified phosphate (APF) or neutral fluoride (NF)) to measure surface roughness (Ra). The material was light-cured using an LED-curing unit (Elipar Freelight Deep Cure-3M/ESPE) for 20 s with a light intensity of 1.200 mW/cm2. Ra measurements were performed before and after treatment with different fluoride gels using a rugosimeter (Mitutoyo SJ210). After Ra, we selected two samples from each group for evaluation using scanning electron microscopy. Data were analyzed using the Shapiro-Wilk, ANOVA, and Tukey tests, with a significance of 5%.
Results:
The average Ra before and after NF did not differ statistically. The average Ra values of the groups treated with APF showed statistically significant differences. The photomicrographs of the groups treated with NF were similar to those of the groups without fluoride treatment. The APF-treated groups showed significant morphological changes.
Conclusion:
NF did not promote changes in the morphology of the evaluated composite resins, in contrast to APF, which caused significant changes.
1. INTRODUCTION
Achieving successful direct restoration depends on surface smoothness, which ensures a comfortable and easy promotion of oral hygiene [1]. Without adequate smoothness, the restoration is susceptible to plaque accumulation, staining of the margins, precocious color change, secondary cavity, and periodontal aggression. Thus, smoothness plays a significant role in improving the appearance and lifespan of restorations [2-5].
Advances in technology have led to the development of nanohybrid and nanoparticle resins with significantly smaller particles to promote greater surface smoothness and satisfactory mechanical properties [6, 7]. The sizes of the particles influence the surface roughness, and the finishing and polishing of composite resin restorations ensure that the surface reaches adequate smoothness [8, 9].
Researchers have shown that both neutral and acidic fluorides may modify the composition and increase the surface roughness of the composite resins, whereas topical application of fluorides can cause alterations in the restoration properties [10]. This can occur due to the interaction of fluoride with reinforcement fillers, filler matrix coupling agents, or the organic matrix [11]. In addition, the surface roughness of composite materials can increase the area of the interfacial surface as well as the thixotropic characteristic viscosity of fluoride gels, both of which can prolong the reaction time of hydrofluoric acid with resin composites [12]. The success achieved during finishing and polishing, in addition to the type of fluoride and restorative material used, determines whether such changes will occur [13, 14].
The aim of this study was to evaluate the effect of applying the gels acidulated phosphate fluoride and neutral fluoride, after finishing and polishing in different types of composite resins. The composite resins used were Z250 microhybrid, Filtek Z350 XT nanoparticle, and Tetric N-Ceram nanohybrid. Surface characteristics were analyzed using surface roughness (Ra) and scanning electron microscopy (SEM) findings. The null hypothesis was that the pH of the fluoride gel would not affect the surface roughness of the composite resins.
2. MATERIALS AND METHODS
2.1. Specimen Preparation
Sixty disk specimens from Z250 (3M-ESPE, St. Paul, MN, USA), Filtek Z350 XT (3M-ESPE, St. Paul, MN, USA), and Tetric N-Ceram (Ivoclar-Vivadent, Schaan, Liechtenstein), and 20 for each type of composite resin, were prepared using a Teflon bipartite matrix (diameter of 5 mm and a height of 2 mm). The resin was applied in a single increment in the matrix, then covered with mylar strips, placed between two glass slabs, and held under pressure for 10 s. The material was light-cured using an LED curing unit (Elipar™ Freelight Deep Cure-3M/ESPE, St. Paul, MN, USA) for 20 s with a light intensity of 1.200 mW/cm2 (Table 1).
The specimens were stored at 37°C for 24 h in a dark vial containing saline to avoid changes in polymerization. Thereafter, the specimens were subjected to the finishing and polishing procedures using the diamond burs 3195 F and 3195 FF (Microdont, São Paulo, SP, Brazil) in that order. We used the cooling, finishing, and polishing discs of Sof-Lex ® (3M-ESPE, St. Paul, MN, USA), silicone Enhance Tips ™ (Dentsply, Milford, DE, USA), and Felt Discs (FGM, Joinville, SC, Brazil) associated with polishing paste Prisma Gloss (Dentsply, Milford, DE, USA) without refrigeration. The polishing movements were performed 20 times, always in the same direction and by a single trained operator. After finishing and polishing, the specimens were cleaned in an ultrasonic bath (30 TD Bio Wash Plus, Bio-art, São Carlos, SP, Brazil) for 8 min and carefully dried with tissue paper towels.
2.2. Roughness Testing
We measured the initial surface roughness measurement (Ra) from three diagonal readings taken for all samples, performed with the analyzer tip in a Mitutoyo SJ 210 rugosimeter (Mitutoyo, Tokyo, Japan) by a single trained examiner, using the following parameters: Ra (mean roughness), corresponding to the arithmetic mean of the absolute values of the roughness profile ordinates (peaks and valleys) in relation to the midline, within the measurement run. At each reading, the rugosimeter needle crossed a 3 mm long area on the surface with a cutoff sampling of 0.25 mm. Acidified phosphate fluoride (1.23%, DFL, Rio de Janeiro, RJ, Brazil) was used for the APF groups and neutral fluoride (DFL, Rio de Janeiro, RJ, Brazil) for the NF groups. Each group contained 10 samples of each composite resin, divided as follows: Z-NF (Z250 resin + neutral fluoride), Z-APF (Z250 resin + acidified phosphate fluoride), XT-NF (Z350 XT resin + neutral fluoride), XT-APF (Z350 resin XT + acidified phosphate fluoride), TC-NF (Tetric N-Ceram resin + neutral fluoride), and TC-APF (Tetric N-Ceram resin + acidified phosphate fluoride), where the NF groups were treated with 2% sodium fluoride gel for 4 min, and APF groups treated with 1.23% acidulated fluoride gel at pH 5 for 1 min.. Excess fluoride gel was removed with a suction cannula. The samples were then stored at 37°C for 24 h, after which the second measurement was performed for surface roughness evaluation. The experimental tests, composite resins, and fluoride therapies used are presented in Table 2.
| Product/ Color/ Manufacturer | Type | Batch No | Composition |
|---|---|---|---|
| Filtek™ Z250/ A2 / 3M ESPE/ | Microhybrid | N335907BR | Zirconia e Silica/0,19-3,3 µm / Bis-GMA, UDMA, Bis-EMA |
| Filtek™ Z350XT/ A2 / 3M ESPE | Nanofilled | N330815BR | Silica/ 20nm/ Zirconia/ 4-11nm/ Bis-GMA, UDMA,TEGDMA, e Bis-EMA |
| Tetric N-Ceram/ A2 / IVOCLAR VIVADENT | Nanohybrid | L58656 | Barium glass, barium aluminium-borosilicate and silica/ Bis-GMA, Bis-EMA, TEGDMA, barium glass |
| Neutral fluoride gel 2% / DFL | Neutral fluoride | L16101442 | 2% sodium fluoride, sodiumsaccharin, hydroxyethyl cellulose, propylene glycol,glycerin, deionized water |
| Phosphate fluoride gel 1.23% / DFL | Acidified phosphate fluoride | L14091446 | Sodium fluoride, cellulose, fluoridric acid, phosphoric acid, propylenoglicol, colorant, flavor, and deionized water |
| Experimental Test | Composite Resin | Fluoride Therapy |
|---|---|---|
| Roughness Scanning electron microscopy |
Z250 | Neutral fluoride 1.23% acidified phosphate fluoride |
| Z350 XT | ||
| Tetric N-Ceram |
2.3. Scanning Electron Microscopy
After the roughness test, two samples from each group were selected for analysis by scanning electron microscopy (SEM, Tescan Mira3, Tescan Ltda, Bmo, Czech Republic). Additionally, two samples of each composite resin with no fluoride treatment were prepared. These samples were dehydrated and gold-sputtered with an Emitech K550 metallizer at a pressure of 2×10-1 mbar and a current of 25 mA for 150 s, resulting in the deposition of a film with an average thickness of ± 15 nm. The samples were mounted on aluminum supports (stubs) using an adhesive tape of carbon metalized with gold. We obtained photomicrographs with magnifications of ×5000 for microstructural analysis.
3. RESULTS
3.1. Roughness Testing
The Tukey test showed no statistically significant difference between initial and final measurements for Z-NF, XT-NF, or TC-NF (NF groups), of these, the TC-NF group was the one that suffered the greatest change between the means obtained before (0.226 μm) and after (0.265 μm) the application of neutral fluoride. However, a significant change in surface roughness was observed between the initial and final measurements in the APF groups (Z-APF, XT-APF, TC-APF) (p < 0.05). The TC-APF group showed the highest mean Ra (0.4810 μm) after the application of the acidulated phosphate gel, and Z-APF had the lowest value (0.3157 μm). The results of the Tukey test are summarized in Tables 3 and 4.
3.2. Scanning Electron Microscopy
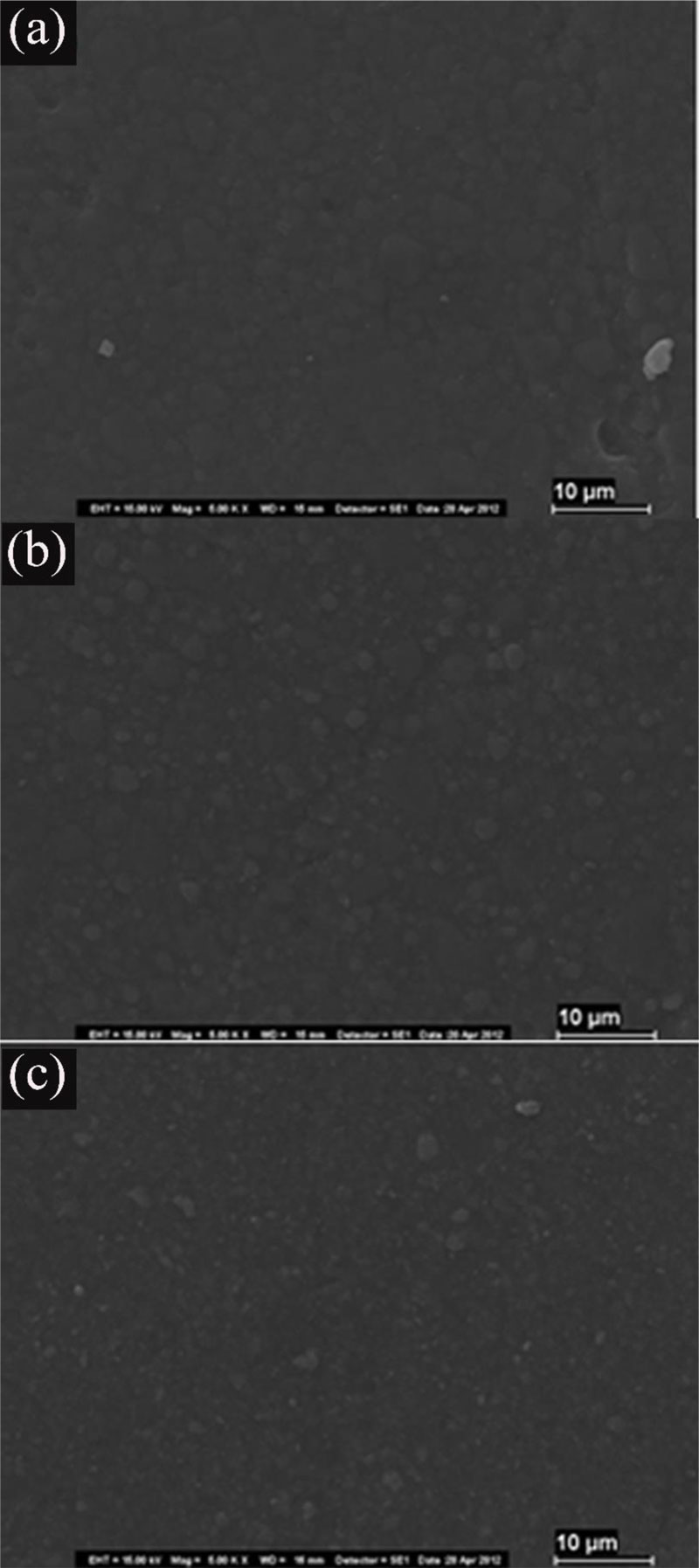
The SEM photomicrographs in Fig. (1) illustrate the samples with no fluoride treatment of the composites stored in saline. The surface was smooth and intact, and the organic matrix was undisturbed.
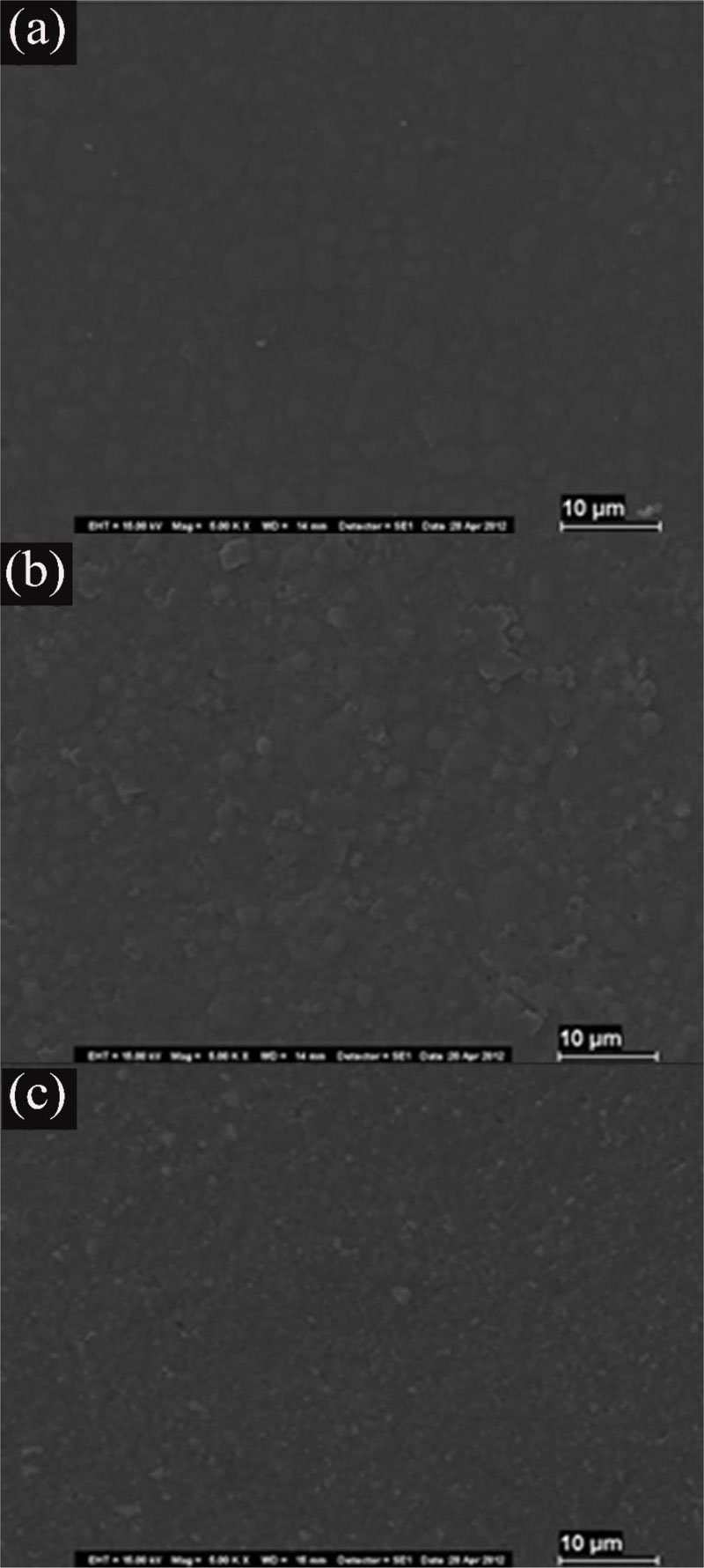
The samples treated with Neutral Gel (NF) (Z-NF, XT-NF, TC-NF) were essentially comparable with the specimens with no fluoride treatment (Fig. 2).
| Composite Resin |
Before NF Mean ± SD |
After NF Mean ± SD |
Difference | P Value |
|---|---|---|---|---|
| Tetric-N-Ceram | 0.226 ± 0.0496 | 0.2650 ± 0.0828 | 0.1360 | Ns |
| Z250 | 0.236 ± 0.0406 | 0.2493 ± 0.0563 | 0.0203 | Ns |
| Z350 XT | 0.2127 ± 0.0422 | 0.2273 ± 0.0432 | 0.0293 | Ns |
| Composite Resin |
Before APF Mean ± SD |
After APF Mean ± SD |
Difference | P Value |
|---|---|---|---|---|
| Tetric-N-Ceram | 0.2922 ± 0.0644 | 0.4810 ± 0.1555 | 0.2057 | < 0,01 |
| Z250 | 0.2663 ± 0.0357 | 0.3997 ± 0.0828 | 0.1700 | < 0,01 |
| Z350 XT | 0.2297 ± 0.0442 | 0.3157 ± 0.0693 | 0.1360 | <0,05 |
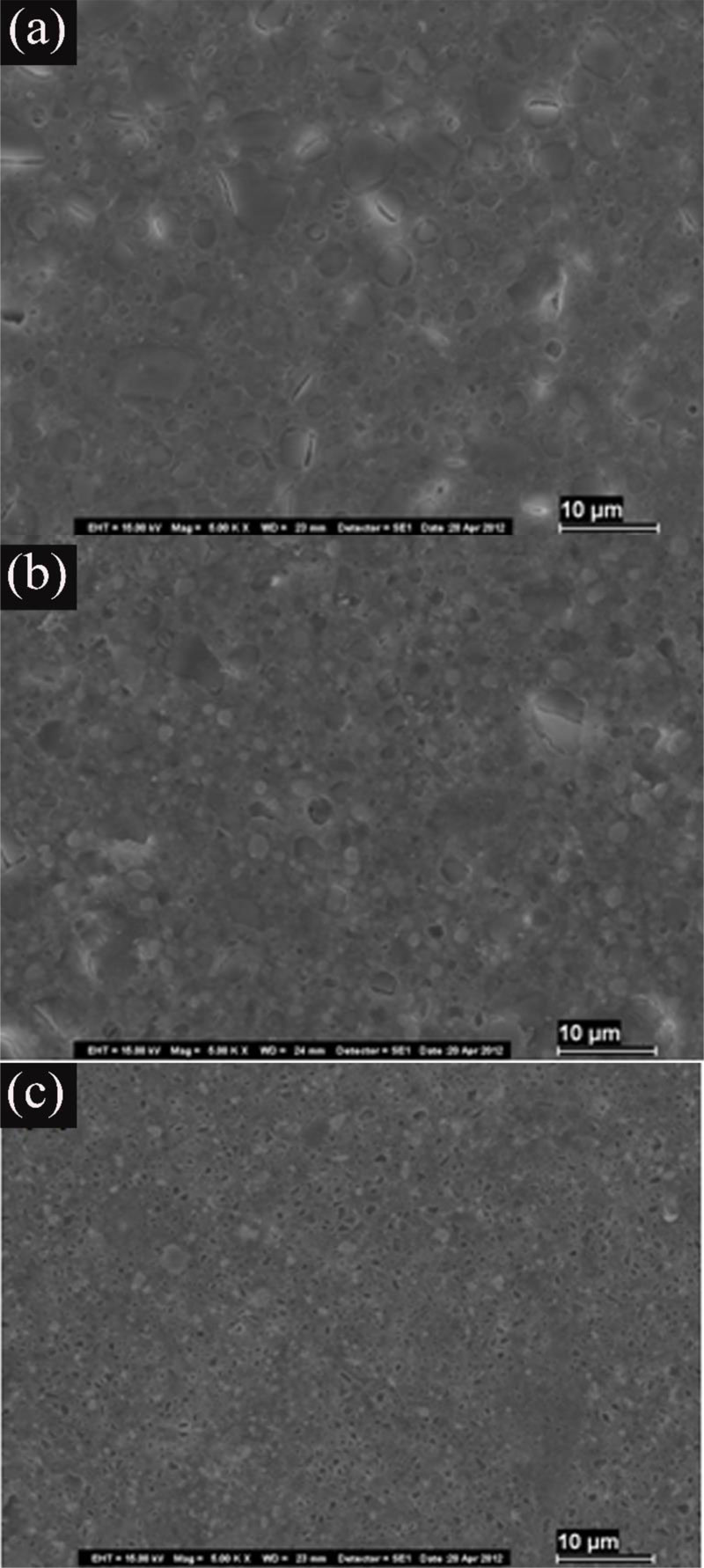
The Z-APF sample (treated with APF gel) showed a smooth surface with cracks, pitting of filler particles, and moderate dissolution of the organic matrix. We noted an abrasive effect, especially on smaller filler particles and the surrounding matrix. Additionally, we observed a tendency for larger filler debonding from the surrounding matrix with cracking after nanocluster dislodgement. The surface appearance of the XT-APF group (treated with APF gel) showed fewer dehydration cracks and matrix dissolution than that of the Z-APF. Moreover, on the TC-APF samples, we noticed a large number of particle voids, in addition to matrix dissolution and eroded particles (Fig. 3).
4. DISCUSSION
We performed the finishing and polishing process before applying fluoride gels. According to Oliveira et al. [10], these materials influence the chemical degradation of the composite resin, as they remove the most superficial layer that is rich in an organic matrix. In addition, changes, including in coloring, surface roughness, shine, microhardness, and wear, depend on time, frequency of application, and the concentration of fluoridated substances [15, 16].
In this study, the null hypothesis was rejected. We analyzed Tetric N-Ceram, Z250, and Z350XT resins treated with APF and NF separately. No significant surface smoothness difference was noted in Z-NF, XT-NF, and TC-NF after treatment with NF, when compared to samples with no fluoride treatment (p>0,05). These results agree with the null hypothesis of this study, which is in accordance with the studies of Golpayegani et al. [17].
Conversely, the resin groups (Z-APF, XT-APF, and TC-APF) treated with APF displayed changes in their structural composition as well as increased surface roughness, thus contradicting the null hypotheses. The degradation in the Z-APF, XT-APF, and TC-APF groups can be explained by the presence of hydrofluoric acid in the composition of the APF, which has a destructive effect on composite resin restorations. This leads to weight loss caused by material wear and integrity loss [14, 15, 18].
SEM studies have demonstrated that changes in composite resin restorations after APF treatment depend on the filler particle size, and that APF has a lesser effect on microparticle composites [17-20]. In this study, the groups Z-APF and TC-APF with nanometric particles in their composition were the most affected by the action of the APF.
Filler particles of composite resins treated with APF showed modifications, which, according to Yip et al. [21] and Al-Samandani [22], may be a result of pH difference and fluoride concentration. The composition of the resin filler particles also influences the degradation caused by fluoride gels. In this study, the TC-APF group displayed the highest rate of filler particle erosion, which can be attributed to their inorganic particles, consisting of barium alumina silicate glass, which, according to Yip et al. [21], are the most affected by APF. The particles present in the other resins in the study, composed of silica and quartz, were less affected by APF.
The XT-APF group experienced less change in the surface after the application of APF, presenting a lower surface roughness. This is attributed to its composition, as it has microparticles in the inorganic portion and is the only test subject with no TEGDMA in its organic portion. As this is a substance susceptible to water sorption [23, 24], it may have facilitated the loss of components by the other two resin composites tested. Conversely, this loss of components may have caused the appearance of dehydration cracks, conserved among the filler particles and the organic matrix, in the Z-APF and TC-APF groups.
Various studies have shown that using APF increases the surface roughness of some composite materials [25-27]. NF has been suggested for patients who have composite resin restorations [28, 29], as the critical surface roughness by bacterial accumulation and colonization on the surface of restorations is 0.2 µm [18].
The surface roughness of composite resin plays an important role in determining how the material interacts with the oral environment [30]. Rough surfaces tend to have less wear resistance and higher friction coefficients than smooth surfaces. In addition, the surface roughness generally determines the mechanical performance of a material. This is because irregularities on the surface are favorable places for the formation of cracks or corrosion [31, 32], as confirmed by the results of this study. Thus, it is unfavorable to proceed with topical fluoride phosphate acidulated in teeth with composite resin restorations.
CONCLUSION
The analysis of Ra and SEM after finishing and polishing the composite resins Z250, Z350XT, and Tetric-N-Ceram showed that the NF did not cause changes in the morphology of the evaluated composites. However, APF promoted changes in morphology in all of the tested composite resins.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The authors confirm that the data supporting the findings of this research are available within the article.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflicts of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors would like to thank the Scanning Electron Microscopy Laboratory at the Museum Paraense Emílio Goeldi.


