Photobiomodulation vs. Placebo on Post-Bleaching Sensitivity and Color Change: A Split-Mouth Clinical Study
Abstract
Objective:
This study aimed to investigate the efficiency of Photobiomodulation (PBM) with low-level LASER therapy compared to placebo in post-bleaching sensitivity and color change during a three-week office bleaching treatment.
Methods:
A split-mouth model was used to evaluate 21 subjects. The right and left hemi-archs were randomized to one of two groups: GP (placebo): simulated LASER application followed by tooth bleaching with 35% hydrogen peroxide; and GL: treated with PBM followed by tooth bleaching with 35% hydrogen peroxide. A four-degree modified Visual Analog Scale (VAS) was used to assess tooth sensitivity after the application of an evaporative stimulus (stimulated pain). In addition, a daily questionnaire was used to measure unstimulated pain. The color change was measured using a spectrophotometer. The Friedman and Wilcoxon tests were used to analyze data sensitivity. Color results were statistically analyzed using Analysis Of Variance (ANOVA) followed by the Tukey post-hoc test.
Results:
There was no significant difference between GP and GL for stimulated pain evaluation (p> 0.05). However, there was a significant difference between the groups for unstimulated pain evaluation (p≤ 0.05). In addition, ΔE data did not reveal any significant difference in tooth color between groups at any time (p> 0.05).
Conclusion:
PBM prevented post-bleaching sensitivity compared to placebo, based on the daily pain assessment questionnaires. PBM did not compromise the quality of bleaching treatments.
1. INTRODUCTION
Post-bleaching sensitivity has been widely reported in the literature; however, it is more frequently observed as a result of in-office tooth bleaching [1-3]. Post-bleaching sensitivity was previously explained due to the penetration of hydrogen peroxide (HP) molecules through dental tissue, which can lead to pulp inflammatory reactions [4, 5] The low molecular weight of HP and its byproducts allows its diffusion through mineralized tissues to reach the pulp chamber [6, 7]
Recent studies have reported several ways to treat sensitivity, aiming to minimize the effects of post-bleaching sensitivity [1, 8-10]. However, no desensitizing agent or forms of treatment have been totally effective or had a long-lasting effect [3, 11, 12]. Low-level laser therapy has been extensively investigated in the literature since 1985 [13]. More recently, low-level laser therapy has been called Photobiomodulation (PBM) due to its biostimulative mechanism of action. However, some doubts remain regarding its use, such as the correct protocol, the use of low or high dosages, and even its association with a desensitizing agent. In addition, almost all clinical trials have evaluated the effect of PBM on dentin sensitivity and not on post-bleaching sensitivity [14]
The use of lasers has been widely described in recent literature for many dental disciplines, such as oral pathology [15, 16], conservative dentistry [17, 18], oral surgery [19], periodontology [20], and orthodontics [21] Low-power lasers have been tested for the treatment of DS using different irradiation protocols. Sgolastra et al.,(2011) [22] and He et al.,(2011) [23] performed systematic reviews on the efficacy of lasers for the treatment of DS. These authors were unable to determine whether the effectiveness of laser treatment was superior to a placebo, but they found that laser application was safe and did not have adverse effects. A recent meta-analysis conducted by Machado et al.,(2018) [24] concluded that more consistent studies should be conducted to draw definitive conclusions about the effect of PBM on DS. To date, no systematic review has investigated the efficiency of PBM in post-bleaching sensitivity. In addition, to the best of the authors’ knowledge, only three randomized clinical trials have investigated the effect of PBM compared with placebos on reducing pain after in-office bleaching [25-27].
In vitro studies have shown important features of PBM’s mechanism of action. The results revealed that their interaction with dental pulp causes a photo-biomodulating effect [28], leading to an increase in the metabolic activity of odontoblastic cells and, as a result, the obliteration of dentinal tubules by means of intensification of the production of tertiary dentin [29]. These possible consequences could interfere with the effectiveness of the bleaching treatment, and no clinical research has been done on this issue. Therefore, this study aimed to evaluate the effect of PBM on post-bleaching sensitivity in relation to placebo as well as the color changes resulting from in-office bleaching.
2. MATERIALS AND METHODS
This study was a randomized, double-blind, placebo-controlled clinical trial with a split-mouth design. The clinical investigation was approved (protocol 2,110,037) by the ethics committee of the local university. The study protocol was registered at https://clinicaltrials.gov under number NCT03514290 and followed the CONSORT statements [30]. All participants signed an informed consent form in full compliance with the Declaration of Helsinki [31].
| Eligibility Criteria | |
|---|---|
| Inclusion Criteria | Exclusion Criteria |
| were aged between 18 to 30 years old | had dentin hypersensitivity |
| had good general and oral health | had congenital enamel defects |
| had caries-free anterior teeth | had visible cracks on enamel |
| had anterior-free restorations | had periodontal disease |
| had canines with shade A2 or darker | had a previous bleaching procedure |
2.1. Eligibility Criteria
The exclusion and exclusion criteria are detailed in Table 1. The subjects chewed pieces of orthodontic rubber to stimulate saliva production. The salivary flow rate was calculated, and the salivary pH was measured using a 510 Benchtop pH meter (Oakton Instruments®; Vernon Hills, Illinois, USA). Patients who presented salivary flow with values between 1 and 2 mLmin and pH between 6.5 and 7.0 were included in the study [8]
2.2. Sample Size
BioEstat program® (Civil Society of Mamirauá, Tefé, AM, Brazil) was used. A pilot study was conducted with 12 subjects following the same protocol used in this clinical study. Statistical power of 80% and an α error of 5% were established for calculation. One point on a scale from 0 to 3 was used to consider a significant decrease in sensitivity.The sample loss rate was 20% at the end of the study. The sample size was 25 patients in total.
2.3. Randomization and Blinding
All subjects received both treatments. In view of this, only the hemi-arch was randomized. A number was assigned to each group: 1 for GP and 2 for GL. A numerical draw was carried out for each volunteer. The first number corresponded to the right hemi-arch and the remaining numbers corresponded to the left hemi-arch [32]. A “block” randomization was performed to avoid unbalanced groups with respect to the two sizes (right or left) at the end of the experiment.
Only one operator (CCS) performed all clinical appointments. A single-blind evaluator (C.M.A.) performed the dental sensitivity assessment of all volunteers. The subjects evaluated in this study did not know which hemi-arch had received PBM. A simulation of the LASER application was performed in the GP, that is, the tip of the equipment was positioned in the same way as in the GL group. However, in the GP, there was no light emission. The noise emitted by the LASER equipment during light emission was simulated using the iTalk Recorder application (Griffin Technology, Nashville, Tennessee, USA) for the iPhone 6 smartphone (Apple®, Cupertino, CA, USA) [27, 32].
2.4. Study Intervention
Prior to PBM, dental prophylaxis was performed using a pumice stone (extra-fine pumice stone, Maquira®; Maringá, PR, Brazil). The right and left maxillary/mandibular quadrants were randomized and allocated to one of two groups: GL: premolars, canines, and incisors of the corresponding hemi-arch received laser irradiation prior to the bleaching treatment; and GP: for premolars, canines, and incisors of the corresponding hemi-arch, the laser tip was positioned similarly to GL but without light irradiation. The GL received PBM by LASER (Photon Laser III, DMC, São Carlos, SP, Brazil), at 808 nm at two perpendicular points using the infrared spectrum, both apical and central on the buccal surface of incisors, canines, and premolars. Emissions to the continuous mode were used; energy of 1.7 J at a dose of 60 J/cm2 was applied to each point for 16 s, with a spot size of 0.028 cm2 [32]. This protocol was carried out in accordance with the manufacturer's recommendations.
After PBM, all patients received the same bleaching treatment. The clinician isolated the gingival tissue surrounding the teeth to be bleached using a light-cured resin dam (Top Dam, FGM®, Joinville, SC, Brazil). Then, tooth bleaching was performed. Four sessions with an interval of seven days were performed, and in each session, three 15-minute applications of 35% hydrogen peroxide gel were performed (Whiteness HP, FGM®, Joinville, SC, Brazil). Peroxide gel was applied to the buccal surface of the incisors, canines, and premolars of the upper and lower hemi-arches. All patients received brushing kits containing a toothbrush (Indicator plus, Oral B®; São Paulo, SP, Brazil) and vegan toothpaste without a desensitizing agent (Natural, Contente®, Uberlândia, MG, Brazil). They were then instructed to use these items three times a day. In addition, volunteers were instructed to avoid acidic foods and drinks (citrus fruits, citrus juices, soft drinks, isotonics, wines, beers, coffees, and teas) throughout the clinical trial.
2.5. Post-Bleaching Sensitivityevaluation
To measure sensitivity after bleaching, a modified Visual Analog Scale (VAS) with an interval of 0 to 3 was used. The scale categories were as follows: absent (0), mild (1), moderate (2), and severe (3). Each score was related to facial expression in illustrative drawings to facilitate patients' perception of pain. Two forms of evaluation were used: stimulated (evaporative stimulus) and non-stimulated pain (questionnaire). The evaporative stimulus was produced using air from a triple syringe (DabiAtlante, Dental Products, Ribeirão Preto, SP, Brazil) that was positioned 1 cm from the tooth and exerted 40 psi of pressure for 5 seconds. Pain sensitivity assessments by means of stimulated pain were performed prior to the bleaching treatment and immediately after each bleaching session. A daily questionnaire was administered during the 21 days of treatment to assess the level of sensitivity caused by the treatment according to the patient's personal perception. The questionnaire was divided into teeth on the right side and teeth on the left side [27].
2.6. Color Evaluation
The color evaluation was performed using an Easyshade Advance 4.0 spectrophotometer (Zahnfabrik, Bad Säckingen, Germany) employing the CIE L*a*b* system. Color change values (ΔE) were obtained using the formula: ΔE = {(ΔL)2 + (Δa)2 + (Δb)2}1/2, where ΔL* = L*- L*0,Δa* = a*-a*0, and e Δb* = b*- b*0 [7]. The evaluation was performed four times: before bleaching treatment (baseline) and after the first, second, and third bleaching sessions. The spectrophotometer equipment was positioned in the central region of each tooth.
2.7. Statistical Analysis
The reported sensitivity levels were analyzed statistically using the BioEstat program® (Civil Society Mamirauity levels were analyz). The Friedman vs. Wilcoxon statistical tests were used. The color change data was employed (using the BioEstat programer the first, and secondly, the patient's personal perception), followed by the Questi test. The level of significance was set at 5%.
3. RESULTS
3.1. Characteristics of the Included Participants
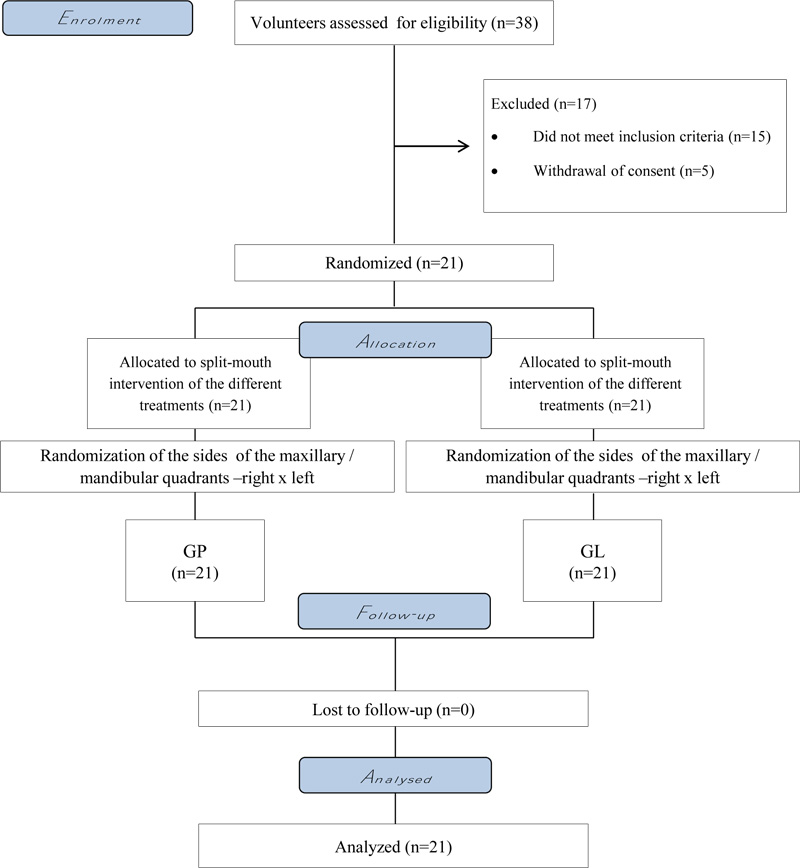
A total of 38 participants were initially evaluated (Fig. 1). The baseline tooth color of the subjects was similar (GP- 4.2 [2.2]; GL- 4.4 [2.6]), and the mean and standard deviation of the subjects' age was 22.1 (5.8) years, ranging from 18 to 30 years. Twelve female participants (57.14%) and 9 male participants (42.86%) were included in the study.
3.2. Evaporative Stimulus Combined with VAS
The results for the different evaluation times are shown in Table 2. There were no significant differences between GP and GL at the different evaluation times (p>0.05). Regarding intra-group analysis, there were also no significant differences between the times of sensitivity assessments in any group (p>0.05).
3.3. Daily Pain Analysis (Questionnaire): 21-Day Follow-up
The results at the different evaluation times are shown in Table 3. For the intergroup analysis, there was a significant difference (p≤0.05) between GP and GL on the days of the bleaching sessions (1stdays, 8th, and 15th day). The lowest sensitivity indices were observed for GL. In the intra-group analysis, both GP and GL presented significant differences (p≤0.05) on the days of the bleaching sessions (1stdays, 8th, and 15th day) when compared to the other evaluated days.
| Groups | Md (±IQR) | |||
|---|---|---|---|---|
| - | Baseline | 1st session | 2nd session | 3rd session |
| GP | 0 (0)Aa | 0 (0)Aa | 0 (0)Aa | 0 (0)Aa |
| GL | 0 (0)Aa | 0 (0)Aa | 0 (0)Aa | 0 (0)Aa |
| Md (±IQR) | |||||||
|---|---|---|---|---|---|---|---|
| 1st week | 1st day | 2nd day | 3rd day | 4th day | 5th day | 6th day | 7th day |
| GP | 2(1)Ab | 0(0)Ba | 0(0)Ba | 0(0)Ba | 0(0)Ba | 0(0)Ba | 0(0)Ba |
| GL | 0.0(1)Aa | 0(0)Ba | 0(0)Ba | 0(0)Ba | 0(0)Ba | 0(0)Ba | 0(0)Ba |
| 2nd week | 8th day | 9th day | 10th day | 11th day | 12th day | 13th day | 14th day |
| GP | 2 (1)Ab | 0(0)Ba | 0(0)Ba | 0(0)Ba | 0(0)Ba | 0(0)Ba | 0(0)Ba |
| GL | 1(1)Aa | 0(0)Ba | 0(0)Ba | 0(0)Ba | 0(0)Ba | 0(0)Ba | 0(0)Ba |
| 3rd Week | 15th day | 16th day | 17th day | 18th day | 19th day | 20th day | 21th day |
| GP | 3(1)Ab | 0(0)Ba | 0(0)Ba | 0(0)Ba | 0(0) Ba | 0(0)Ba | 0(0)Ba |
| GL | 1(1)Aa | 0(0)Ba | 0(0)Ba | 0(0)Ba | 0(0)Ba | 0(0)Ba | 0(0)Ba |
| M (±SD) | |||
|---|---|---|---|
| Groups | 1st session | 2nd session | 3rd session |
| GP | 4.53±(2.4)Aa | 5.44±(4.7)Aa | 5.81±(5.5)Ab |
| GL | 4.80±(3.4)Aa | 5.85±(3.4)Aa | 5.90±(4.4)Ab |
3.4. Color Evaluation
There was no significant difference between the groups (p> 0.05). The intra-group comparison revealed commonality in the two study groups (P <0.05) (Table 4).
4. DISCUSSION
Previous studies have reported a high incidence rate of tooth sensitivity after in-office bleaching procedures in which high concentrations of hydrogen peroxide were used [33-35]. Although generally classified as mild or moderate, the literature shows that sensitivity is a very common symptom following bleaching treatments [2, 7-9]. Hydrogen peroxide has a low molecular weight, which facilitates its penetration into dental tissue and, consequently resulting in tooth whitening. On the other hand, the low molecular weight of hydrogen peroxide promotes the release of inflammatory mediators in the pulp [36, 37].
Many in vitro studies have evaluated the effect of peroxides on dental pulp[38-42].These studies have shown that the application time, the concentration of the bleaching agent, and the type of tooth may be related to pulp inflammation during bleaching. Simultaneously, clinical trials have evaluated the use of anti-inflammatory drugs in the prevention of post-bleaching sensitivity but have not yielded promising results [43, 44]. The use of PBM in the prevention or treatment of post-bleaching sensitivity has been poorly investigated. For this reason, there has been no systematic review of this topic to date. This therapy has analgesic, anti-inflammatory, and biomodulatory effects [45, 46]. The photon energy is converted into chemical energy within the cell, forming ATP, which may lead to increased intracellular Ca+2 [47, 48]. This will ultimately stimulate the modulation of fibroblast growth factor production, which in turn will stimulate cell proliferation [49, 50].This mechanism of action justifies the lower sensitivity reported in GL.
To date, only three clinical trials have evaluated the effect of PBM on post-bleaching sensitivity. The results of the present clinical study are consistent with those obtained by Moosavi et al., (2017) [25] and De Paula et al.,(2018) [27]. However, in the research carried out by Calheiros et al.,(2017) [26], PBM was not effective in the prevention and treatment of post-bleaching sensitivity. Variations in the composition of bleaching gels, especially pH and additives, and the decrease in the pH stability of some products may explain their results [25, 26]. In addition, there is no standardization of the protocol for the use of PBM in dentistry. Furthermore, the small number of clinical trials on this topic makes it difficult to reach a definitive conclusion and makes it impossible to perform a systematic review or meta-analysis on the clinical efficacy of this treatment on tooth sensitivity.
There is no treatment or clinical protocol considered the “gold standard” for the treatment of post-bleaching sensitivity. This makes it difficult for the clinician to make a decision. A recent clinical trial developed by Calheiros et al., (2017) [26] evaluated different protocols for the application of PBM in tooth bleaching. The author evaluated laser treatment before bleaching, after bleaching, and before and after bleaching, and concluded that there was no difference in sensitivity prevention among the three tested treatments. In the present clinical study, PBM was applied immediately before GL bleaching, aiming for a preventive effect.
There was significantly lower sensitivity in GL compared to GP. However, this difference was only detected in the non-stimulated pain (questionnaire), and no significant pain was verified with regard to the stimulated pain. According to Markowitz [7] the nature of sensitivity related to tooth bleaching is different from DS, as explained by Brannstrom [51]. For this reason, the inflammatory characteristics of the bleached tooth could be related to spontaneous pain. Further studies should be conducted to investigate this issue. Although GL presented less sensitivity than GP, light sensitivity was reported in GL. This shows that post-bleaching sensitivity treatment remains a challenge, and further clinical research is encouraged to evaluate different treatments and protocols.
Regarding color change, there was no difference in the quality of the bleaching treatment between GP and GL. This may be because the formation of a tertiary dentin layer may have been compromised by the oxidizing action of peroxide on pulp cells, as demonstrated by some authors in in vitro studies [27, 52]. For this reason, there was no interference of PBM on dentin staining or bleaching.
The modified visual analog scale used in this study was proposed by Thomas Schiff et al. (2009 [53] This VAS is used to assess dentinal sensitivity in many clinical studies (8,10,25). It makes it easier for the patient to classify their pain levels: absent (0), mild (1), moderate (2), and severe [3]. Despite the complexity of post-bleaching sensitivity treatments and the difficulty of establishing adequate methodologies for clinical research, this study showed satisfactory results for PBM. The main limitation of the split-mouth model is the risk that one treatment will affect the other treatment response, an effect known as carry-across [54]. However, there is no consistent evidence that the use of lasers on dental structures can generate any systemic effects on the body. Thus, the carry-across effect likely did not affect the results of this study. In addition, clinical studies depend on patients' discipline and commitment. This question is difficult to address by the researcher.
CONCLUSION
PBM decreased post-bleaching sensitivity compared to placebo when evaluating daily pain using a questionnaire (non-stimulated pain). PBM did not compromise the quality of the bleaching treatment.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the ethics committee of the Federal University of Para , Brazil (protocol 2,110,037). The study protocol was registered at https://clinicaltrials.gov under number NCT03514290.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
All patients signed an informed consent form.
AVAILIBILTY OF DATA & MATERIALS
The data supporting the findings of the article is available in the Plataforma Brasil - Human Research and Ethics Committee of the Institute of Health Sciences of the Federal University of Pará at http://plataformabrasil.saude.gov.br/login.jsf, reference number CAAE: 57534016.0.0000.0018.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


