All published articles of this journal are available on ScienceDirect.
The Effect of Chitosan Nanoparticle as A Final Irrigation Solution on The Smear Layer Removal, Micro-hardness and Surface Roughness of Root Canal Dentin
Abstract
Background:
Root canal irrigation is one of the most important stages during root canal treatment. One of the requirements of root canal irrigation material is that it can eradicate the smear layer but does not alter the physical properties of the root canal dentin.
Objective:
To investigate the effect of chitosan nanoparticle as a final irrigation solution on the smear layer removal, micro-hardness and surface roughness of root canal dentin.
Methods:
Seventy-two premolars used in this study and divided by three evaluations, namely smear layer removal, micro-hardness and surface roughness. Each study used 24 teeth and was assigned randomly into three groups of eight teeth. Group-1, final irrigation with 17% EDTA; group-2, with 0.2% chitosan nanoparticles; group-3, with 2.5% NaOCl. Specimens were evaluated for smear layer removal, micro-hardness and surface roughness using a Scanning Electron Microscope, Vickers hardness tester and surface roughness measuring instrument, respectively. Data obtained from smear layer removal evaluation were statically analyzed using Kruskal-Wallis and Mann-Whitney U and data from micro-hardness and surface roughness were analyzed using one-way ANOVA and Tukey’s test.
Results:
Final irrigation using 0.2% chitosan nanoparticles produced similar smear layer removal than 17% EDTA (P>0.05), but it was greater than 2.5% NaOCl (P<0.05). Chitosan had higher micro-hardness and lower surface roughness than EDTA (P<0.05), but it was the same as 2.5% NaOCl (P>0.05).
Conclusion:
Final irrigation using 0.2% chitosan nanoparticles had the same effect on smear layer removal compared to 17% EDTA; however, 0.2% chitosan produced higher micro-hardness and lower surface roughness of root canal dentin than 17% EDTA.
1. INTRODUCTION
Root canal irrigation is one of the important phases in root canal treatment. The function of root canal irrigation is to remove the smear layers and the remnants of necrotic tissues, as well as to eliminate microorganisms and their products from root canal [1]. Sodium hypochlorite (NaOCl), with a concentration of 2.5-5%, has been frequently employed in the clinic as an irrigation solution. This irrigation solution has the ability to dissolve organic tissues and has antibacterial properties [2]. However, the drawback of NaOCl as an irrigation solution is unable to remove inorganic tissues from the smear layers on the root canal walls [3]. Therefore another irrigation solution needs to be employed as the final irrigation to eliminate inorganic tissues of smear layers. The final irrigation solution is defined as a solution used for eradicating the smear layer thoroughly, especially inorganic components, which remains a presence in the dentin surface of root canal following the organic components have been removed by NaOCl [4].
Ethylenediaminetetraacetic acid (EDTA) is often utilized in the clinic as the final irrigation solution because of its ability to react with calcium ions in dentin forming calcium chelation, thereby dissolving the inorganic tissues from smear layers [3]. However, prolonged exposure of EDTA may alter the structural characteristic of dentin resulting in compromised mechanical integrity and erosion [5]. According to previous investigators [3, 4], the demineralization action of chelating agent probable affects the chemical and physical properties of the structure of root canal dentin, leading to affect micro-hardness and surface roughness. These phenomena may generate the reduction of dentin micro-hardness [6] and induce surface roughness [7] of the dentin of the root canal wall. EDTA also has no antibacterial properties [8]. However, this chelating agent could have benefit in the clinic, as it allows the preparation of narrow root canals and improves the bonding of root canal sealers, which requires dentin surface irregularities for sealer adhesion [9, 10].
Due to the drawbacks of EDTA as a final irrigation solution, hence, other final irrigation solutions need to be studied, which can eliminate both organic and inorganic components of the smear layers, and has antibacterial properties and chelation ability, but no influence on the dentin structures, such as micro-hardness and surface roughness of root canal dentin.
Recently chitosan has been widely used in the health sector [11]. Chitosan is polysaccharide in nature and obtaining from the deacetylation of chitin from the seashell of crustaceans and shrimps [12]. Previous research reported by Silva et al. [13] and Del et al. [14], chitosan has chelation ability; therefore it can dissolve the in-organic parts of smear layers. Chitosan also has an antibacterial effect; hence, it can be considered as a final irrigation solution [14, 15]. Most of the studies on chitosan have focused on the antibacterial property with little literature on smear layer removal, micro-hardness and surface roughness, since these factors could determine the success of root canal treatment. Therefore, the purpose of this study was to investigate the effect of 0.2% chitosan nanoparticles as a final irrigation solution on the smear layer removal and root canal dentin structures, namely micro-hardness and surface roughness.
2. MATERIALS AND METHODS
Seventy-two intact, straight single-rooted mandibular premolars extracted for orthodontic reasons, which have an initial file of # 20 (Dentsply Maillefer, Ballaigues, Switzerland), were used in this study. This study was divided into three evaluations, namely smear layer removal, micro-hardness, and surface roughness, using 24 teeth of each evaluation. The teeth were stored in distilled water and used approximately within one month following extraction. All teeth were sectioned at the cementoenamel junction, leaving the root length of 12 mm, by means of a slow-speed diamond disk with water coolant. The working lengths were deducted 1-mm from the lengths of the roots. The root apices were then closed using the sticky wax to mimic the clinical condition [16].
The root canals were extirpated using barbed broaches (Dentsply Maillefer). The crown-down technique was carried out using a rotary file (Protaper Universal, Dentsply Maillefer) at 250 rpm, up to F3 file reached working length. Root canal irrigation throughout instrumentation and after using each file was undertaken using continuous irrigation technique with 2.5% NaOCl solution (Golden Falcon, Dubai, UAE) (volume of 2 mL, for 1 minute). The root canals were then rinsed with 5 mL of distilled water and randomly divided into three groups of 8 specimens each, according to the final irrigation solution utilized. Group-1 was final irrigated using 5 mL of 17% EDTA (Pulpdent, Watertown, MA, USA) for 3 minutes; group-2, final irrigated with 5 mL of 0.2% chitosan nanoparticle for 3 minutes; group-3, served as a control, using 5 mL of 2.5% NaOCl for 3 minutes.
The 0.2% chitosan nanoparticle solution was made by dissolving 0.2 gram of chitosan powder (size 397.5 ± 98.5 nm) (NHI, Tangerang, Indonesia) in 1% acetic acid with the volume of 100 milliliters. Chitosan was synthesis from shrimp shells (degree of deacetylation >75%) using ionic glass method and Polyanion Tripolyphosphate (TPP) as crosslinker [17]. To obtain a homogenous solution, then the mixture was stirred with a magnetic agitator for two hours [18]. The final irrigation solutions were delivered using a sterile 30-gauge needle, which entered into 2 mm of the working length. All root canals were then rinsed with 5 mL of distilled water, dried with sterile #30 paper points, and sterilized cotton pellets were positioned in the root canal orifices.
2.1. Smear Layer Removal Evaluation
This evaluation used 24 teeth that were assigned into three groups according to the final irrigation solution employed, as stated above. After the application of final irrigations, longitudinal grooves were made on the buccolingual surfaces on each root using a low-speed diamond disk. The roots were then split longitudinally into two halves: buccal and lingual. The root canal was measured and the central part of each apical third was evaluated. The evaluation of smear layer removal was only in the apical third of the root since the penetration of irrigation solution is minimum in the apical third, because of the reduction of diameter and the increase of depth of root canal [19, 20]. The specimens were secured on metal stubs, desiccated and sputter-coated with gold, and the canal wall of the apical third of each root was examined using an SEM (JEOL JSM-5510, Tokyo, Japan). Two calibrated, blinded examiners analyzed all the images, which were obtained at X 1000 and 2000 magnification. The Kappa test was employed to determine the agreement between the examiners (Kappa ≥0.75).
The scoring system on a scale of 1-4 was used to evaluate the degree of removal of the smear layer. Score 1: No smear layer and debris at all, with all tubules cleaned and opened. Score 2: A few areas covered by a smear layer and debris, with most tubules cleaned and opened. Score 3: Smear layer and debris covering almost all the surfaces, with few tubules opened. Score 4: Smear layer and debris covering all the surfaces [21]. Data obtained were analyzed using by Kruskal-Wallis, followed by Mann-Whitney U, with a 95% level of significance.
2.2. Micro-hardness Evaluation
Micro-hardness evaluation used 24 other teeth that were divided into three groups according to the final irrigation solution used, as mentioned above. After the application of final irrigation solutions, each root for the micro-hardness test was sectioned vertically using a cutting saw (Buehler Ltd., Evanston, IL, USA) to the axis of tooth in bucco-lingual direction and obtaining mesial and distal sections. Each root section was embedded in self-curing acrylic resin; the internal surface of root canal dentin was faced up (Fig. 1). The surface was flattened sequentially with 600, 1000, 1500 silicon carbide papers. Subsequently, each specimen was tested using a Vickers Hardness Tester (Shimadzu HMV-2, Shimadzu Corporation, Kyoto, Japan) with a ten-gram load of indenter (15 seconds) (Fig. 2). Three indentations at a distance of 200 µm from each other were done on the apical third of each root [22]. The indentations were viewed on the computer screen connected to the micro-hardness tester. Three indentations then were averaged to determine the value of micro-hardness (in VHN) of each specimen. Data obtained were analyzed statistically using One-Way ANOVA and Tukey’s test with a significance level of 95%.



2.3. Surface Roughness Evaluation
This evaluation used 24 remaining teeth that were assigned into three groups according to the final irrigation solution employed, as stated above. Following the application of final irrigations, all roots were vertically grooved in a bucco-lingual direction and subsequently split into mesial and distal sections. The specimens were positioned on the flat table surface and a clamp was used to hold specimens stable in its position (Fig. 3A). The surface roughness of each sample was tested with a digital roughness tester (SR 300, Taylor Hobson, Leicester, England). The needle of roughness tester was located on the lumen dentin of the root canal and three tracings of different locations on the lumen of root canal dentin were created. The machine was then recording the surface roughness values of root canal dentin. The values, which were expressed as Ra (μm), were exhibited digitally on the screen of the roughness tester (Fig. 3B). The Ra parameter defines the total roughness of the surface and can be described as the numerical average value of the roughness profile from the center line within the measuring length. Data obtained were analyzed by a two-way ANOVA, followed by the Tukey test with a significance level of 95%.
3. RESULTS
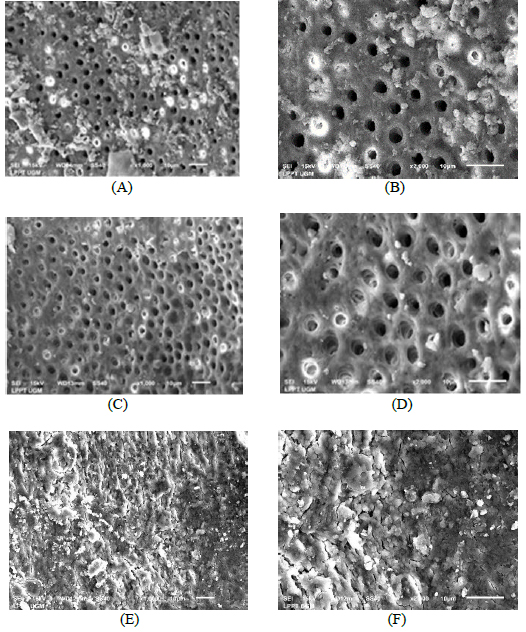
The results of the Kappa tests demonstrated good agreement between two examiners with values of 0.8 or above for all of the different categories. In smear layer evaluation, it can be seen that final irrigations using 0.2% chitosan nanoparticles produced a few areas covered by a smear layer and debris, with most tubules cleaned and opened (Fig. 4A and 4B). No smear layer and debris at all, with all tubules cleaned and opened produced by final irrigation with 17% EDTA (Fig. 4C and 4D). On the contrary, final irrigation using 2.5% NaOCl generated smear layer and debris covering almost all of the surface Fig. (4E and 4F).
Statistical analyzing using Kruskal Wallis showed that final irrigations affected smear layer removal of root canal dentin (p<0.05). The analyzing of Mann-Whitney U revealed that 17% EDTA and 0.2% chitosan nanoparticles caused greater smear layer removal than 2.5% NaOCl (P <0.05), while 17% EDTA and 0.2% chitosan nanoparticle generated the same effect on smear layer removal (P > 0.05) (Table 1).
| - | Smear Layer Removal | ||
| 0.2% chitosan nanoparticle | 17% EDTA | 2.5% NaOCl (Control) |
|
| Median | 2 | 1 | 3 |
| Kruskal Wallis | 10.93 | ||
| P | 0.004 | ||
| Mann-Whitney U | a | a | b |
Micro-hardness study (Table 2) demonstrated that 0.2% chitosan nanoparticles produced greater micro-hardness than 17% EDTA (P < 0.05), but was less than 2.5% NaOCl (P > 0.05). One-way ANOVA showed a significant difference occurred among groups of micro-hardness evaluations (P < 0.05).
In surface roughness evaluation (Table 3), it was exhibited that EDTA produced the greatest surface roughness than other final irrigation solutions, whereas chitosan nanoparticles had lower surface roughness than EDTA, but was higher than NaOCl. A one-way ANOVA revealed that a significant difference occurred among three final irrigation solutions. (P < 0.05). Tukey’s test demonstrated that a significant difference occurred in surface roughness between 17% EDTA compared to either 0.2% chitosan nanoparticles or 2.5% NaOCl (P <0.05). In contrast, there was no significant difference in surface roughness between 0.2% chitosan nanoparticles and 2.5% NaOCl (P > 0.05).
| Micro-hardness (VHN) | |||
| 0.2% chitosan nanoparticle | 17% EDTA | 2.5% NaOCl (Control) |
|
| Mean ± SD | 49.88 ± 2.34 | 45.04 ± 4.02 | 50.72 ± 2.08 |
| ANOVA: F | 8,09 | ||
| P | 0.002 | ||
| Tukey’s | a | b | a |
| - | Surface Roughness (µm) | ||
| 0.2% chitosan nanoparticle | 17% EDTA | 2.5% NaOCl (Control) |
|
| Mean ± SD | 0.84 ± 0.23 | 2.41 ± 0.09 | 0.74 ± 0.21 |
| ANOVA: F | 236.61 | ||
| P | 0.000 | ||
| Tukey’s | a | b | a |
4. DISCUSSION
The results of this study indicate that 0.2% of chitosan nanoparticle had the same effect on the smear layer removal as 17% EDTA. Final irrigation using both solutions seem to dissolve the smear layer, especially the inorganic substance, although in the different mechanism [15, 23]. EDTA has chelation property since it can create chelation with calcium ions in dentin and causing the dentin to dissolve. EDTA has the capability to decalcify dentin with a depth of 20-50 μm ranging between two to three minutes [24]. Similar to EDTA, chitosan also has chelation properties. Although not entirely understanding its effect on dentin, it has been presumed that adsorption, ion exchange and chelation control the establishment of interaction between the chelating agents and the metal ions [25]. Additionally, ion involvement, chitosan chemical structure, and solution pH may specify the interaction type between chelating substances and metal ions [22, 26].
Two theories attempt to elucidate the chelating effect of chitosan. One theory is known as the “chemical chain bridge model”, which explains that two or more amino groups of chitosan chain attach to a similar metal ion. The second theory is named “the hook or free-arm model”, which mentions that only one amino group of the material structure is involved in the attachment, which is the metal ion attached to the amino group. The chitosan polymer is formed by a chain composed of several chitin dimers. This chitin dimer exhibits two nitrogen atoms with pairs of free electrons liable for the interaction between the metal and the chelating substance. In an acid environment, the amino groups exist in the protonated of bi-polymer, leading to a complete position charge (-NH3+). This system facilitates binding with other molecules, causing the occurrence of adsorption [5, 27, 28].
In this study, final irrigation using 2.5% NaOCl produced the lowest smear layer removal but caused the highest micro-hardness and the lowest surface roughness compared to chitosan and EDTA. This finding is in accordance with other studies exhibiting that NaOCl is not effective in removing the inorganic part of the smear layers [29] and removed smear layers only on the superficial of the root canal dentin [26, 30, 31].
Previous investigators reported that chitosan has many advantageous properties, for example, bio-compatible, bio-degradation, bio-adhesion, and non-toxic to human cells [32]. Moreover, it is also extensively available in nature, cheap, and has chelating properties of metal ions, which was verified in this study [33]. Therefore, chitosan has been developed for final irrigation solution in endodontic field, which in the future, can replaced EDTA, which has several drawbacks. The size of chitosan, which is in nanoparticles, also influence the penetration of this irrigation solution deeper into the tubules of the root canal system [14, 34]. Chitosan polymer is hydrophilic, which favors intimate contact with root canal dentin; as a result, it is adsorbed easily to root canal walls and delivered to the deeper location of dentinal tubules [34]. Additionally, it has a large number of free hydroxyl and amino groups that lead to the ionic interaction between dentin calcium ions and the chelating agent. The efficiency of a chelating agent also relies on several factors, such as application time, pH, the concentration of the solution and amount of solution [35]. Thus, in the present study, the volume of chitosan (pH 6) used for final irrigation was standardized at 5 mL for 3 minutes [13].
Application of final irrigation solution, especially using a chemical solution, may also lead to the changes of the root canal dentin structures, such as micro-hardness and surface roughness. This condition might be associated with the demineralizing effect of the chelating solutions on root canal dentin. A change in surface structures occurs after using this chelating solution since this solution has a demineralizing effect on the dentinal walls leading to a decrease in micro-hardness and an increase in surface roughness [36-38].
The results of this study proved that EDTA had an effect on micro-hardness of root canal dentin, and produced the lowest micro-hardness than chitosan and NaOCl (P < 0.05). Previous research with Atomic Absorption Spectrophotometry also showed that irrigation with chelation agents could remove calcium ions from the root canals [6] Chelation and demineralization agents not only dissolve the inorganic structure in the smear layer but also dissolve the calcium hydroxyapatite matrix from dentin; hence collagens open and micro-hardness reduced [8, 22]. EDTA has a strong chelation action, which may be due to its greater capability to demineralize the smear layer, especially inorganic parts. This demineralization effect may lead to erosion, especially peritubular and intertubular dentin, as a result, enlarge the dentinal tubules and weaken dentin, as well as alter the surface of dental hard tissues [25]. This phenomenon occurred due to the change in the ratio of Calcium/Phosphorus in dental tissue [13], which lead to a decrease in micro-hardness and an increase in surface roughness of root canal dentin.
In contrast, 0.2% of chitosan nanoparticles had smaller changes in the structure of dental hard tissues compared to EDTA. These results verify that chitosan is a weak chelating substance that demineralizes less dentin surface than EDTA. Therefore, 0.2% chitosan nanoparticles solution has been capable of removing the smear layer, but not in inducing dentin demineralization [22]. In addition, chitosan, which makes contact with dentin surface, is likely to induce remineralization of demineralized dentin. The covalent interaction of chitosan to collagen of dentin has been assumed to create the remineralization of demineralized dentin. This phenomenon arises due to the groups of phosphate that may attract calcium ions to make a satisfactory surface for nucleation of crystals, resulting in the occurrence of calcium-phosphate layer [23].
Moreover, chitosan might improve the dentinal surface degradation by collagenase [28]. Therefore, the remineralization ability of chitosan may explain why chitosan had higher micro-hardness and lower surface roughness compared to EDTA [35]. This study also revealed that NaOCl produced the greatest micro-hardness and lowest surface roughness. It can be explained that NaOCl is only dissolving organic materials and generating dentin collagen denaturation and dissolution. However, this solution is not as effective as final irrigation due to not effectively remove the inorganic materials of the smear layer. Besides, NaOCl has no capability to induce erosion to root canal dentin [14].
CONCLUSION
It can be concluded that the final irrigation using 0.2% chitosan nanoparticles had the same effect on smear layer removal compared to 17% EDTA; however 0.2% chitosan produced higher micro-hardness and lower surface roughness on root canal dentin than 17% EDTA. Chitosan has been developed for irrigation solution in the endodontic field, which in the future, can replace EDTA, which influence the root canal dentin structures.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
We confirm that we used teeth specimens which were obtained from the laboratory for study purpose and this study is not directly on the human subjects.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available in the repository UGM at https://repository.ugm.ac.id, reference number 4467.
FUNDING
This study was funded by Universitas Gadjah Mada, Faculty of Dentistry Grant number 4467/UN1/KG/Set.KG/ LT/2018.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors would like to thank to Mrs. Nofa Mardia Ningsih Kaswati for providing Chitosan from Nanotech Herbal Indonesia.


