All published articles of this journal are available on ScienceDirect.
Obesity and Periodontal Disease: A Narrative Review on Current Evidence and Putative Molecular Links
Abstract
Background:
Obesity represents one of the main health problems worldwide and is considered a risk factor for several diseases, including periodontitis, which is a microbially-associated inflammatory disease affecting the tooth-supporting structures.
Objective:
The aim of this review was to report the current direct and indirect evidence concerning the possible association between obesity and periodontitis and their putative molecular links.
Methods:
A literature search was conducted between January 1999 and September 2019, in PubMed/MEDLINE and Science Direct databases, using pertinent keyword combined by Boolean operators. Through a multi-step screening process (literature search; articles title and abstract evaluation and full-text reading), studies fitting inclusion/exclusion criteria were considered for the review.
Results:
35 studies were included in the present review (17 observational studies; 7 systematic reviews; 11 systematic reviews with meta-analysis), focusing on the direct and indirect evidence of the possible association between obesity and periodontitis and their potential etiopathogenic molecular links
Conclusion:
Although the majority of the studies reported a positive association between obesity and periodontitis, the heterogeneity of the classification criteria and of the clinical parameters employed in the studies for both obesity and periodontitis evaluation, complicated the comparison of the results, thus considered inconclusive. Although several putative molecular pathogenic links between obesity and periodontitis have been highlighted, further studies, with longer follow-ups and with homogeneous clinical criteria, are needed to better understand the putative relation between obesity and periodontal disease.
1. INTRODUCTION
Obesity represents one of the main health problems worldwide, with a prevalence triplicated in the last 40 years [1, 2] and estimated between 2015 and 2016, of 13.9% for US children (2-5 years old), 18.4% in school-aged children (6-11 years old), 20.6% in adolescents (12-19 years old), and 39.8% in adults [3].
Obesity is defined as an excessive body fat accumulation, due to the imbalance between the food eaten and the calories spent [4], and is evaluated through Waist Circumference (WC) [5] and Body Mass Index (BMI) [6] assessment.
Waist circumference measurement is applied for cardiovascular diseases risk prediction, indicating: a moderate risk, with values ≥ 94 cm in men and ≥ 80 cm in women; a severe risk, with values ≥ 102 cm in men and ≥ 88 cm in women; an extremely high risk, with values ≥ 120 cm in men and ≥ 110 cm for women [7].
BMI calculation, obtained by dividing a person’s weight by height square (Kg / m2), identifies overweight with values between 25 and 29.9, while, values ranging from 30 to 34.9, from 35 to 39.9, and over 40, indicate a Slight or First Degree of obesity, a Moderate or Second degree of obesity, and a Severe or Third degree of obesity, respectively [6]. BMI, is the most common measure for obesity screening, even though it is not able to differentiate lipids distribution neither in somatic and visceral fat or in relation to age, sex and race [8].
Obesity is the result of many disorders arising from a number of different causes, including complex interactions among genetic, biochemical, neural and psychological factors, on one side, and environmental, social and economic factors, on the other side [9]. Moreover, obesity is considered itself a risk factor for several diseases, such as type 2 diabetes mellitus, cardiovascular disease, tumors and, potentially, for periodontal disease [1].
Periodontitis is an inflammatory microbially-associated disease, leading to the destruction of the tooth-supporting structures [10, 11]. It is likely associated with diabetes, cardiovascular disease, cancer, respiratory infections, metabolic syndrome and, according to recent evidence, to obesity too [12, 13].
Since the first report suggesting in a rat model the existence of a possible relation between obesity and periodontal disease in 1977 [14], only 21 years later, Saito et al. conducted the first human study on the Japanese population, revealing that obese subjects showed a probability of suffering from periodontitis 8.6 times greater than non-obese ones [15].
The present review aims to report current higher-level evidences relating obesity to periodontal disease and the putative molecular pathogenic links.
2. METHODS
2.1. Search Methods
A literature search was independently conducted by two reviewers through PubMed / MEDLINE and Science Direct pertinent keywords, combining databases, using the following them with the three Boolean operators AND, OR, NOT, for each search in the above-mentioned databases:
1. Periodontitis
2. Periodontal diseases
3. Gingivitis
4. Periodontal inflammation
5. 1 OR 2 OR 3 OR 4
6. Obesity
7. Overweight
8. 6 OR 7
9. CAL (acronym of Clinical Attachment Level) AND 8
10. PPD (acronym of Periodontal Probing Depth) AND 8
11. BoP (acronym of Bleeding on Pobing) AND 8
12. 9 OR 10 OR 11
13. BMI (acronym of Body Mass Index) AND 5
14. Waist to Hip ratio AND 5
15. Periodontal treatment AND 8 OR 12 OR 13
16. Bariatric Surgery AND 5 OR 12
17. Weight Loss AND 5 OR 12
18. 15 OR 16 OR 17
19. CRP (acronym of c-Reactive Protein) AND 5 OR 8 OR 12 OR 18
20. Oxidative stress AND 5 OR 8 OR 12 OR 18
21. IL-1b AND 5 OR 8 OR 12 OR 18
22. TNF-a AND 5 OR 8 OR 12 OR 18
23. IL-6 AND 5 OR 8 OR 12 OR 18
24. IL-8 AND 5 OR 8 OR 12 OR 18
25. Inhibitor of the plasminogen activator AND 5 OR 8 OR 12 OR 18
2.2. Inclusion and Exclusion Criteria
Cross-sectional and longitudinal studies, systematic reviews and meta-analysis, in the English language, in press or published in scientific journals, between January 1999 and September 2019, were included in the present study.
Literature reviews, case reports and case series studies, together with conference papers and oral communications, edited books and book chapters, were excluded from the present study, together with cross-sectional and longitudinal studies, systematic reviews and meta-analysis not published in English language or published before January 1999.
3. RESULTS
3.1. Studies Screening Process
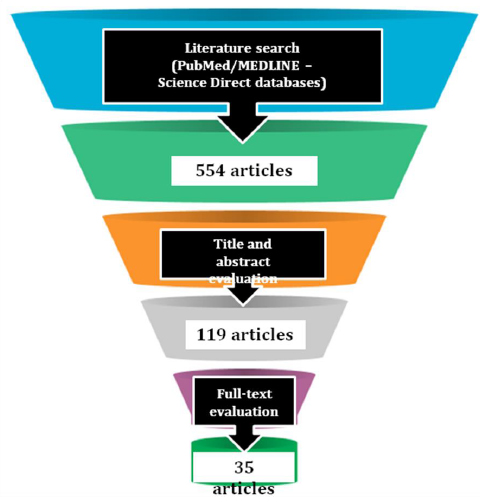
From literature research, 554 articles were identified; out of these, subsequently to articles title and abstract evaluation, 119 were considered eligible for the present review; after the full-text evaluation, 35 articles, fitting the above-mentioned inclusion and exclusion criteria, were included in the present study, as shown in Fig. (1).
3.2. Studies Included in the Present Review
Among the 35 articles considered in the present study: 17 reported observational studies (6 cross-sectional, 13 case-control and 8 pro-/retro-spective studies, respectively); 7 were systematic reviews; 11 were systematic reviews with meta-analysis. Included studies type is reported in (Table 1).
3.3. Studies Features
Two systematic reviews with meta-analysis, together with 2 longitudinal and 1 cross-sectional studies, analyzed the direct evidence of the possible association between obesity and periodontitis; in addition, 3 systematic reviews and 1 systematic review with meta-analysis evaluated the possible influence on such a relationship of age and gender, and 2 systematic reviews and 1 systematic review with meta-analysis of the smoking habit.
The indirect evidence of the possible association between obesity and periodontal disease derived from 2 systematic reviews with meta-analysis, investigating the impact of bariatric surgery and weight loss on periodontal status, on the one side, and 2 systematic reviews and 1 systematic review with meta-analysis, reporting the effect of the periodontal treatment on body weight and obesity, on the other side.
Twenty-five observational studies (5 cross-sectional, 14 case-control and 6 pro-/retrospective studies, respectively), 3 systematic reviews and 7 systematic reviews with meta-analysis focused on the potential etiopathogenic and molecular links between obesity and periodontitis, evaluating also the role of: CRP, oxidative stress, IL-1b, TNF-a, IL-6, IL-8, and the inhibitor of plasminogen activator. Included studies features are shown in Table 1.
3.4. Summary of the Results
The direct evidence of the possible association between obesity and periodontal disease, also in relation to gender, and smoking habit, was not conclusive, except for Chaffee et al. who reported a stronger association between obesity and periodontitis in women when compared to men. Studies evaluating the effect of bariatric surgery on periodontal status, as well as the influence of overweight/obesity on periodontitis development, also reported contrasting results, weakening the indirect evidence of the possible relationship between obesity and periodontitis.
Conversely, Keller et al. and Suvan et al hypothesized that overweight and obesity may affect periodontitis onset and worsening and several studies highlighted the bi-directional role of several inflammatory response markers (C-Reactive Protein, Nitric Oxide, Myeloperoxidase), pro-inflammatory cytokines and adipokines (Interleukin-1b; Tumour Necrosis Factor alpha; Interleukin-6; Interleukin-8; Plasminogen-1 activator inhibitor) in such a possible association.
4. DISCUSSION
4.1. Direct Evidence of the Possible Association between Obesity and Periodontal Disease
Kongstad et al. reported, in a cross-sectional study, that obese subjects had lower Clinical Attachment Level (CAL) values, measured in mm as the distance between the cementoenamel junction and the periodontal pocket bottom [16], but an higher predisposition for bleeding, when compared to normal-weight subjects, neither if smokers or non-smokers [17].
Saxlin et al. concluded, in a longitudinal study, that BMI and WC values appeared to be associated with the number of teeth showing Probing Pocket Depth (PPD) values, measured in mm as the distance between the free gingival margin and the periodontal pocket bottom [16], ≥ 4 mm; such results could not be considered conclusive due to the study limits, such as randomness, restriction in enrolled patients age range (45-64 years old) and lack of anamnestic records (e.g. smoking habit; systemic conditions; etc.) [18].
Similarly, Chaffee et al. reported a slight positive association between obesity and periodontal disease, since both higher CAL values were registered in obese compared to normal-weight subjects and higher BMI values were observed in subjects affected by periodontal disease compared to subjects with healthy periodontal conditions [19]; the relation between overweight or obesity and tooth loss was not confirmed in the studies reviewed by the authors, in contrast with Nascimento et al. findings, who subsequently found a positive association between tooth loss and obesity in all meta-analyses included in their systematic review [20].
De Castilhos et al. observed, in a cohort study, an association between BMI and WC with PPD, CAL, Bleeding on Probing (BOP) and Plaque Index (Pl) [21, 22].
4.1.1. Age and Gender Influence
A positive association between obesity and periodontal disease in adults as well as in adolescent subjects was reported in a review by Khan [23].
Gender influence on the association between overweight or obesity and the development of periodontal disease is still controversial. Martinez-Herrera et al. [24] and Keller et al. [25] could not achieve conclusive results, while Chaffee et al. reported a stronger association between obesity and periodontal disease in women when compared to men [19].
4.1.2. Smoking Habit Effect
Chaffee et al. in their systematic review reported a stronger association between obesity and periodontal disease in non-smokers subjects compared to the general adult population [19].
Conversely, AlJehani et al. in a review and Keller et al. in a systematic review stated that, in the putative relation between obesity and periodontal disease, smoking habit has not been widely investigated, and may be a possible confounder because of both the negative effect exerted on the periodontal health and the positive effect on the body weight [25, 26].
| Authors | Year | Title | Type of the Article | Topic(s) |
|---|---|---|---|---|
| Kongstad et al. | 2009 | The relationship between body mass index and periodontitis in the Copenhagen city hearth study | Cross-sectional | Direct evidences of the possible association between obesity and periodontitis |
| Saxlin et al. | 2010 | Overweight and obesity weakly predict the development of periodontal infection | Longitudinal | Direct evidences of the possible association between obesity and periodontitis |
| Chaffee et al. | 2010 | Association between chronic periodontal disease and obesity: A systematic review and meta-analysis | Systematic review + meta-analysis | Direct evidences of the possible association between obesity and periodontitis + Age and Gender influence (etc.) |
| Nascimento et al. | 2016 | Is there a relationship between obesity and tooth loss and edentulism? A systematic review and meta-analysis | Systematic review + meta-analysis | Direct evidences of the possible association between obesity and periodontitis |
| De Castilhos et al. | 2012 | Association between obesity and periodontal disease in young adults: A population-based birth cohort | Longitudinal | Direct evidences of the possible association between obesity and periodontitis |
| Khan et al. | 2018 | Is overweight/obesity a risk factor for periodontitis in young adults and adolescents?: A systematic review | Systematic review | Age and Gender influence on the possible association between obesity and periodontitis |
| Martinez-Herrera et al. | 2017 | Association between obesity and periodontal disease. A systematic review of epidemiological studies and controlled clinical trials | Systematic review | Age and Gender influence on the possible association between obesity and periodontitis |
| Keller et al. | 2015 | Association between periodontal disease and overweight and obesity: A systematic review | Systematic review | Age and Gender (etc.) + Smoking habit influence on the possible association between obesity and periodontitis + Overweight/obesity effect on periodontitis onset and worsening |
| Fontanille et al. | 2018 | Bariatric surgery and periodontal status: A systematic review with meta-analysis | Systematic review + meta-analysis | Indirect evidences of the possible association between obesity and periodontitis |
| De Souza et al. | 2018 | Relationship between bariatric surgery and periodontal status: A systematic review and meta-analysis | Systematic review + meta-analysis | Indirect evidences of the possible association between obesity and periodontitis |
| Papageorgiou et al. | 2015 | Effect of overweight/obesity on response to periodontal treatment: systematic review and a meta-analysis | Systematic review + meta-analysis | Indirect evidences of the possible association between obesity and periodontitis |
| Suvan et al. | 2000 | Periodontal complications with obesity | Systematic review | Indirect evidences of the possible association between obesity and periodontitis + Overweight/obesity effect on periodontitis onset and worsening |
| Gerber et al. | 2016 | Influence of obesity on the outcome of non-surgical periodontal therapy - A systematic review | Systematic review | Indirect evidences of the possible association between obesity and periodontitis |
| Moura-Grec et al. | 2014 | Obesity and periodontitis: systematic review and meta-analysis | Systematic review + meta-analysis | Overweight/obesity effect on periodontitis onset and worsening |
| Suvan et al. | 2011 | Association between overweight/obesity and periodontitis in adults. A systematic review | Systematic review | Overweight/obesity effect on periodontitis onset and worsening |
| Furugen et al. | 2008 | The relationship between periodontal condition and serum levels of resistin and adiponectin in elderly Japanese | Cross-sectional | Potential etiopathogenic and molecular links between obesity and periodontitis + Inhibitor of the plasminogen activator + Adiponectin + Leptin |
| Genco et al. | 2005 | A proposed model linking inflammation to obesity, diabetes and periodontal infections | Cross-sectional | Potential etiopathogenic and molecular links between obesity and periodontitis |
| Vivekannada et al. | 2019 | Impact of Weight Reduction on Adiponectin and TNF-α Levels in the Gingival Crevicular Fluids of Obese Patients with and without Periodontal Disease | Case-control | Potential etiopathogenic and molecular links between obesity and periodontitis |
| Dos Santos et al. | 2019 | Clinical periodontal conditions in individuals after bariatric surgery: A systematic review and meta-analysis | Systematic review + meta-analysis | Potential etiopathogenic and molecular links between obesity and periodontitis |
| Nascimento et al. | 2015 | Is weight gain associated with the incidence of periodontitis? A systematic review and meta-analysis | Systematic review + meta-analysis | Potential etiopathogenic and molecular links between obesity and periodontitis |
| Wilkins et al. | 2017 | Influence of Obesity on Periodontitis Progression Is Conditional on Interleukin-1 Inflammatory Genetic Variation | Longitudinal | Potential etiopathogenic and molecular links between obesity and periodontitis + IL-1 |
| Kopp et al. | 2003 | Impact of weight loss on inflammatory proteins and their association with the insulin resistance syndrome in morbidly obese patients | Cross-sectional + Longitudinal | Potential etiopathogenic and molecular links between obesity and periodontitis + CRP |
| Visser et al. | 1999 | Elevated C-reactive protein levels in overweight and obese adults | Longitudinal | Potential etiopathogenic and molecular links between obesity and periodontitis + CRP |
| Cairo et al. | 2008 | Severe periodontitis in young adults is associated with sub-clinical atherosclerosis | Case-control | Potential etiopathogenic and molecular links between obesity and periodontitis |
| Higashi et al. | 2008 | Periodontal infection is asso- ciated with endothelial dysfunction in healthy subjects and hypertensive patients | Case-control | Potential etiopathogenic and molecular links between obesity and periodontitis |
| Paraskevas et al. | 2008 | A systematic review and meta-analyses on C-reactive protein in relation to periodontitis | Systematic review + meta-analysis | Potential etiopathogenic and molecular links between obesity and periodontitis |
| Nam et al. | 2018 | Salivary biomarkers of inflammation and oxidative stress in healthy adults | Cross-sectional | Potential etiopathogenic and molecular links between obesity and periodontitis + CRP |
| Dezayee et al. | 2016 | Saliva C-reactive protein as a biomarker of metabolic syndrome in diabetic patients | Case-control | Potential etiopathogenic and molecular links between obesity and periodontitis + CRP |
| Keaney et al. | 2003 | Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study | Cross-sectional | Potential etiopathogenic and molecular links between obesity and periodontitis |
| Virgolici et al. | 2005 | A comparative oxidative stress study – obesity with and without diabetes mellitus | Case-control | Potential etiopathogenic and molecular links between obesity and periodontitis |
| Uzun et al. | 2004 | Changes in leptin, plasminogen activator factor and oxidative stress in morbidly obese patients following open and laparoscopic Swedish adjustable gastric banding | Case-control | Potential etiopathogenic and molecular links between obesity and periodontitis + Oxidative stress |
| Cherian et al. | 2019 | Malondialdehyde as a Marker of Oxidative Stress in Periodontitis Patients | Longitudinal | Potential etiopathogenic and molecular links between obesity and periodontitis + Oxidative stress |
| Saita et al. | 2016 | Novel antioxidative nanotherapeutics in a rat periodontitis model: Reactive oxygen species scavenging by redox injectable gel suppresses alveolar bone resorption | Longitudinal | Potential etiopathogenic and molecular links between obesity and periodontitis + Oxidative stress |
| Takane et al. | 2002 | New biomarker evidence of oxidative DNA damage in whole saliva from clinically healthy and periodontally diseased individuals | Case-control | Potential etiopathogenic and molecular links between obesity and periodontitis + Oxidative stress |
| da Silva et al. | 2017 | Genetic Factors and the Risk of Periodontitis Development: Findings from a Systematic Review Composed of 13 Studies of Meta-Analysis with 71,531 Participants | Systematic review | Potential etiopathogenic and molecular links between obesity and periodontitis + IL-1 |
| Stadler et al. | 2016 | Gingival crevicular fluid levels of cytokines/chemokines in chronic periodontitis: A meta-analysis | Systematic review | Potential etiopathogenic and molecular links between obesity and periodontitis + IL-1 + IL-8 |
| Joshi et al. | 2014 | Association between obesity-related adipokines and colorectal cancer: A case-control study and meta-analysis | Case-control | Potential etiopathogenic and molecular links between obesity and periodontitis + TNF-a and IL-6 + Adiponectin + Resistin |
| Joshi et al. | 2014 | Obesity related adipokines and colorectal cancer: A review and meta-analysis | Systematic review + meta-analysis | Potential etiopathogenic and molecular links between obesity and periodontitis + TNF-a and IL-6 + + Resistin |
| Zimmermann et al. | 2013 | Local and circulating levels of adipocytokines in obese and normal weight individuals with chronic periodontitis | Case-control | Potential etiopathogenic and molecular links between obesity and periodontitis + TNF-a and IL-6 |
| Akram et al. | 2017 | Cytokine Profile in Chronic Periodontitis Patients with and without Obesity: A Systematic Review and Meta-Analysis | Systematic review + meta-analysis | Potential etiopathogenic and molecular links between obesity and periodontitis + IL-8 |
| Akram et al. | 2016 | Resistin as potential biomarker for chronic periodontitis: A systematic review and meta-analysis. | Systematic review + meta-analysis | Potential etiopathogenic and molecular links between obesity and periodontitis + IL-8 + Resistin |
| Yamaguchi et al. | 2007 | Adiponectin inhibits osteoclast formation stimulated by lipopolysaccharide from Actinobacillus actinomycetemcomitans | Cross-sectional | Potential etiopathogenic and molecular links between obesity and periodontitis + Adiponectin |
| Karthikeyan et al. | 2007 | Leptin levels in gingival crevicular fluid in periodontal health and disease | Case-control | Potential etiopathogenic and molecular links between obesity and periodontitis + Leptin |
| Karthikeyan et al. | 2007 | Gingival crevicular fluid and serum leptin: their relationship to periodontal health and disease | Case-control | Potential etiopathogenic and molecular links between obesity and periodontitis + Leptin |
| Gonullu et al. | 2010 | Association between adiponectin, resistin, insulin resistance, and colorectal tumors | Case-control | Potential etiopathogenic and molecular links between obesity and periodontitis + Resistin |
| Suresh et al. | 2018 | Effect of nonsurgical periodontal therapy on plasma-reactive oxygen metabolite and gingival crevicular fluid resistin and serum resistin levels in obese and normal weight individuals with chronic periodontitis | Case-control | Potential etiopathogenic and molecular links between obesity and periodontitis + Resistin |
| Salman et al. | 2019 | Study of changes of obesity-related inflammatory cytokines after laparoscopic sleeve gastrectomy | Longitudinal | Potential etiopathogenic and molecular links between obesity and periodontitis |
| Patel et al. | 2014 | Gingival crevicular fluid and serum levels of resistin in obese and non-obese subjects with and without periodontitis and association with single nucleotide polymorphism at -420 | Case-control | Potential etiopathogenic and molecular links between obesity and periodontitis + Resistin |
Similar results were later reported by Reibel et al. (2003) and Bergstrom et al. (2004), showing that smokers had a higher prevalence of periodontal disease and worse PPD values and furcation defects compared to non-smokers [3, 27].
Martinez-Herrera et al. [24] in a recent review, concluded that smoking habit predisposes to periodontal disease and that smoking habit and obesity are independent risk factors for periodontal disease.
4.2. Indirect Evidence of the Possible Association between Obesity and Periodontal Disease
The indirect evidence of the possible association between obesity and periodontal disease derived totally from 4 systematic reviews with meta-analysis and 1 systematic review with meta-analysis, is discussed below.
4.2.1. Impact of Bariatric Surgery and Weight Loss on Periodontal Health Conditions
Fontanille et al. reported in their systematic review and meta-analysis a periodontal health status worsening 6 months after bariatric surgery, therefore recommending periodontal screening procedures even before bariatric surgery to avoid a postoperative worsening [28].
Conversely, de Souza et al. [29] concluded that bariatric surgery may improve the periodontal health status, and mainly the plaque index, in obese patients probably promoting behavioral changes, metabolic control of glycemic level, and reducing inflammatory mediators.
4.2.2. Impact of Periodontal Treatment on Obesity
In the systematic review results of Papageorgiou et al., after the periodontal treatment, no statistically significant differences were found between obese and normal-weight in relation to most of the periodontal parameters [30]
Suvan et al. reported in a systematic review inconsistent evidence of different clinical responses to non-surgical periodontal therapy in obese subjects compared to nonobese ones [30, 31].
Conversely, Gerber et al. concluded in their systematic review that obesity may be associated with poorer periodontal health and might also decrease the therapeutic response to non-surgical periodontal treatment [32].
4.3. Potential Etiopathogenic and Molecular Links between Obesity and Periodontitis
A strong relation between obesity and periodontal disease was reported by a systematic review, stating that obese subjects were significantly more prone to develop periodontal disease compared to non obese ones [33].
Moreover, Keller et al. in their systematic review, including many longitudinal studies, some of them with a 20 years follow-up, suggested that overweight, obesity, weight gain, and increased WC may be considered as risk factors for periodontal disease development and for periodontal parameters worsening [25].
Suvan et al. in a systematic review reported a positive relation between obesity and periodontitis [31], confirmed in a very recent review, showing that obese subjects were more prone to periodontal disease onset compared to normal-weight subjects, regardless of age and gender [34]
The potential mechanism underlying the putative mutual relationship between obesity and periodontitis may be related to low-grade inflammation.
The periodontal disease itself determines an increase in the circulating levels of pro-inflammatory cytokines, that contribute to the worsening of chronic systemic disorders [35], on one side, and the higher levels of cytokines secreted by the adipose tissue in overweight and obese subjects may affect local and systemic inflammation caused by periodontal disease, on the other side.
This bi-directional mechanism may be responsible for the increased levels of the inflammatory response markers, such as C-Reactive Protein (CRP), Nitric Oxide (No) and Myeloperoxidase (MPO), as well as of the pro-inflammatory cytokines secreted by the adipose tissue, such as Interleukin-1b (IL-1b); Tumour Necrosis Factor alpha (TNF-a); Interleukin-6 (IL-6); Interleukin-8 (IL-8); and Plasminogen-1 activator inhibitor [33, 36]. In addition, adipose tissue secretes also hormone-like proteins, called adipokines, such as Leptin, Adiponectin and Resistin; It is noteworthy that some of these play an important role in periodontal disease too [36-40].
4.3.1. C-Reactive Protein
C-Reactive Protein is considered one of the main inflammatory markers [41].
Its serum concentration resulted higher in obese people compared to normal weight ones [31, 42]. Kopp et al., similarly to Visser et al., reported that increased serum levels of C-Reactive Protein were associated with serum IL-6 ones, released by the adipose tissue in larger amounts in obese compared to non obese subjects [43, 44].
In addition, serum CRP levels have been found higher in subjects suffering from periodontitis compared to periodontally healthy ones [45, 46]; these levels decreased after the periodontal therapy [47]. CRP was also detected in the saliva as well as in the gingival crevicular fluid of subjects affected by the periodontal disease [48, 49].
4.3.2. Oxidative Stress
It has been proposed that obesity may affect chronic disorders through both the inflammatory state and the alteration of the oxidative state [50, 51].
Virgolici et al. observed that the plasmatic parameters of oxidative stress were altered in obese subjects compared to normal-weight ones [52]. Moreover, weight loss has been related to decreased oxidants formation [53].
Reactive oxygen species can be produced also in periodontitis [54], as proposed by Krol et al., who assessed that the oxidative stress can lead to the formation of lesions in periodontal tissue [55]. Lipid peroxidation is one of the main events associated with inflammation in periodontal tissue [56].
4.3.3. IL-1b
IL-1 is a pro-inflammatory cytokine active in immune response against microbial agents. IL-1 induces chemokines and prostaglandins secretion, together with metalloproteinases production, enhancing, in turn, tissue destruction [57].
IL-1b role in periodontal disease pathogenesis is well-established and its genetic variation is considered as a risk factor for PD [41, 58]. Indeed, it has been hypothesized that only in obese subjects with IL1b polymorphism, the systemic inflammation related to obesity may affect PD progression, alternatively, it has been proposed that subjects genetically predisposed to IL-1b over-expression may be more susceptible to visceral adiposity formation and to PD development and severity [41].
4.3.4. TNF-a and IL-6
TNF-a and IL-6, proinflammatory cytokines released by adipose tissue and immune cells, are detected in higher serum concentrations in obese compared to nonobese subjects, with a tendency to decrease with weight loss [59, 60].
Higher serum TNFa concentration has been reported in obese periodontally healthy subjects compared to obese subjects affected by PD, suggesting the existence of a relation between TNFa and obesity independently of the periodontal status, while increased serum IL6 levels resulted in subjects affected simultaneously by obesity and PD, indicating a synergistic role of these pathologic conditions in serum IL6 levels [61].
On the other hand, higher TNFa concentrations were reported in gingival crevicular fluids of overweight and obese subjects compared to normal-weight ones, collected from sites affected by both gingivitis and PD and may partially derive from the adipose tissue. This finding may indicate a putative negative effect exerted by obesity on PD, with crevicular TNFa elevation and subsequent connective tissue and bone destruction [61].
4.3.5. IL-8
IL-8 is associated with the inflammation of the initial periodontal lesion and with chronic periodontitis [58]; however, no significant difference in IL-8 levels was found between obese and normal-weight subjects suffering from periodontal disease [62, 63].
4.3.6. Inhibitor of the Plasminogen Activator
Inhibitor of the plasminogen activator secretion, involved in autocrine and paracrine signaling, promotes other chronic diseases, such as diabetes, cardiovascular disease and periodontal disease onset and/or worsening [36].
Inhibitor of the plasminogen activator serum levels and leptin levels have been reported to be reduced in the bloodstream from obese subjects [64].
4.3.7. Adiponectin
Adiponectin, produced by the adipose tissue, is a 30-kDa polypeptide, increasing insulin sensitivity and decreasing circulating fatty acids [36].
Adiponectin exerts both an anti-inflammatory effect, mainly antagonizing TNF-a, and an anti-cancer effect, inhibiting cell growth and angiogenesis, together with pro-inflammatory cytokines secretion [59].
It has been found to inhibit osteoclast formation induced by periodontopathic bacteria Lipopolysaccharides [65] and subsequent alveolar bone loss; in this perspective, adiponectin may be considered as a protective factor for PD [36].
The observation that adiponectin serum levels are reduced in type 2 diabetes, as well as in obesity, may explain the reduced protective effect even towards PD [36].
4.3.8. Leptin
Leptin is a 16-kDa non-glycosylated peptide, produced by adipose tissue. Its serum levels correspond to adipose tissue total amount, resulting in increased levels in obese subjects. It is also produced by pre-adipocytes, under the paracrine stimulation of the surrounding macrophage-derived cytokines, especially IL-1, IL-6, TNF-a, and of the bacterial Lipopolysaccharides [36].
Leptin receptors are expressed on adipocytes and also on vascular endothelial cells and T lymphocytes, suggesting a possible role in immune response, possibly reducing regulatory T CD4+ lymphocytes. In compliance with this hypothesis, leptin may favour periodontal tissue inflammation interfering with T cells activity [36].
Higher levels of serum Leptin have, indeed, been detected in periodontopathic patients. Conversely, gingival crevicular leptin concentrations resulted lower in periodontopathic sites compared to healthy ones probably due to leptin local consumption in the inflamed sites [66, 67].
4.3.9. Resistin
Resistin is an adipokine and it is secreted from adipose tissue and monocytes/macrophages [68], involved in the inflammatory process, affecting the secretion of IL-6, TNF-α and Adiponectin [59, 60, 69].
Resistin serum level has been found to increase in several chronic inflammatory conditions, such as obesity, diabetes and rheumatoid arthritis [70].
Similarly, resistin was found consistently elevated in subjects suffering from periodontal disease [63], as confirmed by Patel et al., who reported the association between periodontal inflammation and higher resistin levels [71].
CONCLUSION
Although the majority of the studies reported a positive association between obesity and periodontitis, the heterogeneity of the classification criteria and of the clinical parameters employed in the studies, for both obesity and periodontitis evaluation, complicated the comparison of the results, thus considered inconclusive. The use of the Classification of Periodontal and Peri-Implant Diseases and Conditions proposed in the 2017 World Workshop [10-12] in the future studies might make studies results uniform and thus simpler to compare one another.
Most of the studies reported that the potential pathogenic mechanism underlying the relationship between obesity and periodontitis may rely on the shared and potentially inter-related low-grade inflammation, involving several cytokines, adipokines and oxidative stress. Anyway, although several putative molecular pathogenic links between obesity and periodontitis have been highlighted, further studies, with longer follow-ups are needed to better understand the putative relation between obesity and periodontal disease, in order to possibly pave the way for novel biomarkers for obesity and periosontitis and for innovative preventative strategies and special recommendation for both obese and periodontal subjects.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
All the authors have contributed to the study, being involved in literature search, drafting and revising the manuscript.


