Immunosuppressive Effect of Mesenchymal Stromal Cells is Enhanced by IL-1α from Oral Squamous Cell Carcinoma Cells
Abstract
Background:
We have already reported that mouse Oral Squamous Carcinoma Cells (OSCCs) Sq-1979 specifically enhance the immunosuppressive activity of mesenchymal 10T1/2 cells via the functional soluble factor (s).
Objective:
In this report, we attempted to identify soluble factor(s) mediating the immunosuppression of Sq-1979 cells.
Methods:
L5-11 cells are a variant established from the metastatic lymph nodes of Sq-1979-implanted mice. Unlike parental Sq-1979 cells, however, L5-11 cells lack promotion of immunosuppressive activity in 10T1/2 cells. In order to identify cytokine mRNAs specifically expressed in Sq-1979 cells but not in L5-11 cells, cDNA microarray was performed. Conditioned medium from Sq-1979 cells (CM) was absorbed by several different neutralizing antibodies (abs) against the corresponding cytokines. The absorbed CM was then co-cultured with 10T1/2 cells and anti-CD3 antibody-stimulated mouse spleen cells. The Interferon (IFN) -γ producing capability of the stimulated spleen cells was evaluated using Enzyme-Linked Immunosorbent Assay (ELISA). By using a specific cytokine product instead of CM in this co-culture system the source of the immunosuppressive effect was identified.
Results:
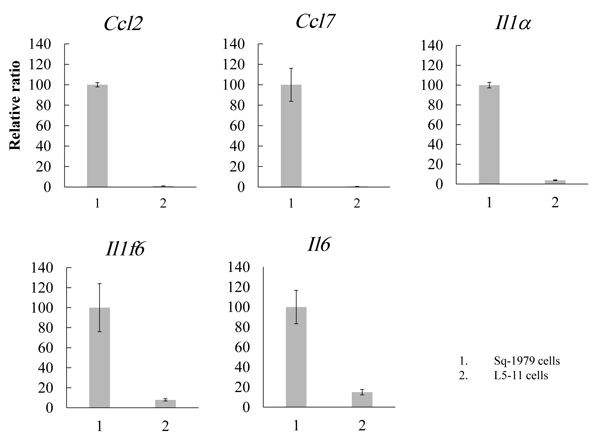
The expression of Ccl2, Ccl7, Il1-α, IL1f6 and Il6 mRNAs was specifically elevated in Sq-1979 cells compared to L5-11 cells. The suppression of the IFN-γ producing capability of stimulated spleen cells in the co-culture system was specifically alleviated by absorbing the CM with anti-IL-1α ab. We further demonstrated that the immunosuppressive effect of CM in the co-culture system could be completely substituted by IL-1α protein (50 pmol/ ml).
Conclusion:
The immunosuppressive function of 10T1/2 cells is specifically promoted by IL-1α, secreted by Sq-1979 cells.
1. INTRODUCTION
OSCC is the most common malignant tumor of the oral cavity [1]. The disease has historically had a poor prognosis, with a low overall survival rate of about 60% [2] attributed to recurrence and cervical lymph node metastasis. Yet despite its prevalence and severity, the 5 year survival rate has not been improved for the past 30 years [3]. The onset of OSCC has also been linked to chronic inflammation resulting from periodontitis. Research has suggested that chronic infection of resident microbiota P. gingivalis activates enzymatic cascades enhancing cellular invasion by OSCCs [4]. The progression and malignancy of tumors, including oral squamous cell carcinoma (OSCC), is regulated by the biological influences of tumor micro environments (TME) surrounding the tumor tissue that affect the host’s immunological response [5-7]. Several lines of evidence have demonstrated that stromal cells surrounding tumor cells play important roles in developing progressive phenotypes of tumor tissues. In head and neck cancers, cancer associated fibroblasts (CAFs) have been suggested to promote angiogenesis, invasion and Treg induction via several cytokines including IL-6, CCL2, CCL7, TGF-β1 and TNF-α [8]. CAF-educated macrophage progenitor cells reduce T cell proliferation via TGF-β1, IL-10 and ARG1, suggesting that CAFs can induce protumoral Tumor-Associated Macrophages (TAMs) in the immunosuppressive microenvironment [9]. In OSCC tissues, CAFs are divided into 3 grades on the basis of the expression of alpha smooth muscle actin, with the CAFs in the highest grade promoting CD163 positive macrophages, which are associated with poor prognosis [10]. Constitutive production of IL-1α from OSCCs enhances IL-6 production of CAFs [11]. IL-6 and GM-CSF, produced by pancreatic CAFs, are important mediators of Myeloid Derived Suppressor Cell (MDSC) differentiation [12].
In the TME, infiltration of CD8+ T cells is an indicator for good prognostic criterion [13]. We have already reported that the regulatory mechanism of antitumor immunity mediated by Th1 cytokine, IFN-γ, is drastically changed among the mice implanted with primary versus advanced variants of the OSCC cells [14]. Our results further suggest that the immunosuppressive function of mesenchymal stromal cells is specifically enhanced by humoral factor(s) from primary OSCC cells [15], which, however, do not induce any MDSCs in the tumor-bearing mice [16]. In contrast, in the advanced OSCC-bearing mice, MDSCs could possibly be a major conductor of immunosuppression [16]. Therefore, our results strongly suggest that the immune-modulatory functions of OSCC can be uniquely regulated according to their malignant stages. In the early stage, mesenchymal stromal cells (i.e., CAF) could be a unique effector. Humoral factor(s) from OSCC cells force CAF to exert immune suppression via the direct cell contacts to effector T cells [15].
In this report, we attempted to isolate the humoral factor(s) secreted by OSCC cells in order to potentiate CAF-mediated immune suppression that enables the progression of OSCC.
2. MATERIALS AND METHODS
2.1. Experimental Animals
Twenty-four-week-old male C3H/HeN mice were purchased from Chubu Kagaku Shizai Co., Ltd. (Nagoya, Japan) and maintained ad libitum on Oriental MF solid chow (Oriental Yeast Co., Tokyo, Japan).
2.2. OSCC Cells and Sub-Clones
The C3H mouse OSCC cell line, Sq-1979, and C3H mouse embryotic fibroblasts, 10T1/2 cells, were obtained from the RIKEN BioResource Center (Ibaraki, Japan). Sq-1979 cells were grown in Eagle’s minimum essential medium (E-MEM; Wako, Osaka, Japan) supplemented with 10% fetal bovine serum (FBS, NICHIREI BIOSCIENCES INC., Tokyo, Japan) and 1% Pen Strep (penicillin 10,000 unit/mL, streptomycin 10,000 µg/mL; Gibco®, Life Technologies, Grand Island,NY,USA). 10T1/2 cells were grown in Basal Medium Eagle (BME; Gibco®, Life Technologies) supplemented with 10% FBS (NICHIREI BIOSCIENCES INC.) and 1% Pen Strep (Gibco®, Life Technologies). Establishments of L cells including L5-11, a metastatic Sq-1979 sub-clone established from the tumor-implanted mice, were described [17].
2.3. RNA Extraction and Microarray Analysis
RNA extractions were performed using ISOGEN (Nippon Gene, Tokyo, Japan), according to the manufacturer’s instructions. Total RNAs were extracted from Sq-1979 and L5-11 cells. cDNA microarray analysis was performed (Oncomics, Nagoya, Japan) using the Superscript G3 mouse GE Microarray 60K kit (Agilent, Santa Clara, CA, USA). To ensure the reliability of the data, genes were considered to be differentially expressed if the P-values were < 0.001 (Student t-test) and had a fold change > 2.0.
2.4. Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR).
Whole-cell RNA extraction and quantitative PCR was performed as previously described [13]. Primer sequences were designed by Primer Express software (version 2; Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Primer sequences were as follows: Ccl2, forward 5'-AAA AAC CTGG ATC GGA ACC AAA TG-3' and reverse 5'-TGC TTG AGG TGG TTG TGG AAA AG-3'; Ccl7, forward 5'- TGC TCA TAG CCG CTG CTT TCA G-3' and reverse 5'- GCT TCC CAG GGA CAC CGA CTA C-3'; Il1α, forward 5'- TTT GAC ATG TAT GCC TAC TCG TCG G-3' and reverse 5'- CTG TGA TGA GTT TTG GTG TTT CTG GC-3'; Il1f6, forward 5'- AAG AAC TAA GGC TGC TCT GGC TTT CC-3' and reverse 5'- TGC CAT GGT CCT TAT CTC TCA ATG A-3'; Il6, forward 5'- CCT ACC CCA ATT TCC AAT GCT CTC-3' and reverse 5'- GCA TAA CGC ACT AGG TTT GCC G-3'; ribosomal protein S5 (RPS5), forward 5'- AGA AGA CTC AAC ACG CAT TGG GC -3' and reverse 5'- GCA CTC AGC GAT GGT CTT GAT GT -3'. Each expression level of mRNA was normalized to RPS5.
2.5. Preparation of Mouse Spleen Cells
Spleens were removed from more than twenty four-week-old male C3H/HeN mice, with the spleen cells isolated as described [18]. Briefly, the spleen tissue was mashed with a stainless steel mesh in Roswell Park Memorial Institute (RPMI) 1640 medium (Sigma-Aldrich, St. Louis, MO) containing 10% FBS (NICHIREI BIOSCIENCES INC.), 50 µM 2-mercaptoethanol (Nacalai Tesque, Kyoto, Japan) and 1% antibiotic antimycotic solution (penicillin 10,000 unit/mL, streptomycin 10,000 µg/mL, amphotericin B 25 µg/mL; Gibco®, Life Technologies). Cells were collected by centrifugation at 1,500 rpm for 5 min, and then red blood cells in the collected cells were removed using a red blood cell lysis buffer (10 mM Tris-HCl (pH7.3) containing 140 mM NH4Cl and 1mM Na2EDTA). The spleen cells were washed and re-suspended with the RPMI-1640 medium and filtered using a cell strainer (Falcon®, Corning Inc., NY, USA) to remove the residue.
2.6. OSCC Cell-Conditioned Medium
To confirm humoral factor(s) from Sq-1979 cells, conditioned medium (CM) was prepared from cultured Sq-1979 cells. One million Sq-1979 cells were cultured in 10 mL of RPM-I1640 medium supplemented in 5% CO2 at 37°C. The CM was collected after 36 h by centrifugation at 1,000 rpm for 5 min. As a control, RPMI medium was denoted as untreated medium (UM). The concentration of IL-1α in the CM was assayed by sandwich ELISA using Mouse IL-1α /IL-1F1 Quantikine ELISA Kit (R&D Systems Inc., Minneapolis, MN, USA). For standardization, a calibration curve against re combinant mouse IL-1α was run with each assay. Finally, in order to absorb specific cytokines, CM was pre-treated with various combinations of 1µg/ml mouse anti CCL2 (AF-479-NA), CCL7 (AF-456-NA), IL-1α (AF-400-NA), IL-6 (AF-406-NA) and IL-1F6 (AF2296) antibodies (R&D systems) at 4°C for 8h. To confirm cytokine activity, recombinant mouse IL-1α (400-ML-005, R&D systems) was used.
2.7. Analysis of IFN-γ Production from Spleen Cells which were Co-Cultured with CM and/or 10T1/2 Cells
The spleen cell suspension (4 × 105/well) was added to a 96-well plate (3599, Corning), on which 1 μg/mL of anti-CD3 monoclonal antibody (mAb) (145-2C12 BD Biosciences, San Diego, CA, USA) had been immobilized (0.1 mL/well) at 4°C overnight. Then, CM (diluted to 50% in the final culture medium) and/or 10T1/2 cells (3 × 103/well) were added to the wells and co-cultured with the spleen cells in the RPMI 1640 basal medium supplemented with 10% FBS for 48 h in 5% CO2 at 37°C. After that, the supernatant was harvested by centrifugation at 3,000 rpm for 5 min and stored at − 80°C.
The concentration of IFN-γ in the supernatant of the cell culture was assayed by sandwich ELISA using BD OptE1A set (BD Biosciences). For standardization, a calibration curve against recombinant mouse IFN-γ was run with each assay.
In each experiment, stimulated spleen cells were cultured without 10T1/2 in UM as a control. For intra-examiner agreement, the values of the control culture were checked and were within the specified range (300~500 ng/mL IFN-γ produced).
2.8. Western Blot Analysis
Cells were scraped with a rubber policeman and collected in 10× cell lysis buffer (Cell Signaling Technology, Beverly, MA, USA). A solution containing phenylmethanesulfonyl fluoride (1 mM) plus one tablet of protease inhibitor cocktail (Complete, EDTA-free; Roche Diagnostics GmbH, Mannheim, Germany) was added to each cell lysate. Protein contents in the lysates were assayed, and equal amounts of protein for each sample were subjected to 15% SDS-polyacrylamide gel electrophoresis, followed by immunoblotting with primary antibodies against anti-IL-1α rabbit polyclonal antibody (ab9724) (Abcam PLC, Cambridge, UK, 1:1,000) and anti-β-actin antibody (Sigma-Aldrich, St.Louis, Mo, USA, 1:10,000). Signals were detected using the corresponding peroxidase-conjugated secondary antibodies (anti-rabbit IgG or anti-mouse IgG; Cell Signaling Technology and used at a concentration of 1:10,000), and immunoreactive bands were visualized by chemiluminescence (Clarity™ Western ECL substrate; Bio-Rad, Hercules, CA, USA). The membranes and images were developed with an ImageQuant™ LAS500 Imaging System (GE Healthcare Bio-Sciences AB, Uppsala, Sweden).
3. RESULTS
3.1. Isolation of Cytokine mRNAs Predominantly Expressed in Sq-1979 Cells.
The results of our previous study suggest that humoral factor(s) secreted from Sq-1979 cells could specifically transmit a suppressive function against the IFN-γ-producing capability of antigen-stimulated spleen cells in co-culture with 10T1/2 cells. By contrast, the activity of the stimulated spleen cells was not reduced in the co-culture containing L cells, a metastatic Sq-1979 cell variant [15]. Therefore, the suppressive activity of 10T1/2 cells was specifically enhanced by humoral factor(s) generated by Sq-1979 cells but not by L cells. In order to identify cytokine mRNAs specifically expressed in Sq-1979 cells, cDNA micro array analysis was performed between mRNAs from Sq-1979 and L5-11 cells. We eventually focused on the 5 mRNAs Il1-α, Il1f6, Il6, Ccl2 and Ccl7. As shown in Fig. (1), the expression of these mRNAs was predominantly elevated in Sq-1979 rather than in L5-11 cells.
3.2. Blocking Immunosuppressive Function of CM by Neutralizing Antibodies against Cytokines.
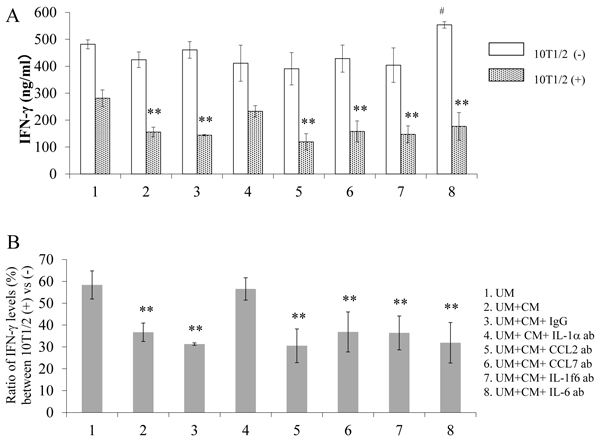
As shown in Fig. (2A), the IFN-γ-producing capability of the stimulated spleen cells (lane 1, 10T1/2(-)) was significantly reduced in the presence of directly contacted 10T1/2 cells (lane 1, 10T1/2(+)), with further reductions caused by the addition of Sq-1979 conditioned medium (CM) (lane 2 of 10T1/2(+)). The capability of CM to reduce IFN-γ production was negated by absorbing the CM with anti-IL-1α antibody (ab) (lane 4 of 10T1/2(+)). However, it remained unchanged when absorbed by anti-CCL2, CCL7, IL-1f6 and IL-6 abs (lanes 5, 6, 7 and 8 of 10T1/2(+)), or by the addition of IgG, as a control antibody (lane 3 of 10T1/2(+)). On the other hand, in the absence of 10T1/2 cells, IFN-γ-producing capability was not reduced by treating the cells with CM (lane 2 of 10T1/2(-)) or any of its absorbed variants (lanes 1 through 8 of 10T1/2(-)).
As shown in Fig. (2B), the ratio of IFN-γ-producing capability was reduced to almost 60% in the presence of 10T1/2 cells (lane 1), and further reduced to less than 40% by adding CM (lane 2). This CM mediated reduction was completely negated by absorbing the CM with anti-IL-1α ab (lane 4). However, it remained unchanged when absorbing the CM with anti-CCL2, anti-CCL7, anti-IL-1f6, or anti-IL-6 abs (lanes 5-8, respectively). The CM mediated reduction was also unchanged by the addition of control IgG (lane 3).
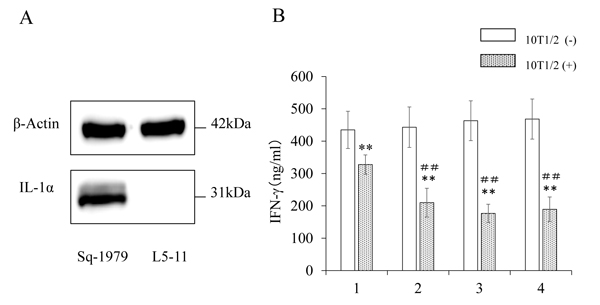
3.3. Promotion of Immunosuppressive Activity of 10T1/2 by IL-1α
We examined the expression of IL-1α protein in OSCC cells. As shown in Fig. (3A), Sq-1979 cells expressed significantly high levels of IL-1α, while the expression was scarcely detectable in L5-11 cells. Using ELISA, we also found that the concentration of IL-1α in CM of Sq1979 cells was 52.5 ± 1.3 pg/ ml (n=3). Thus, we concluded that IL-1α was specifically produced and secreted in the CM by Sq-1979 cells. In order to examine the functional significance of the IL-1α in CM, recombinant IL-1α was mixed with UM, then added to the co-culture of stimulated spleen cells and 10T1/2 cells. As shown in Fig. (3B), the reduced IFN-γ-producing capability of the stimulated spleen cells in the presence of 10T1/2 cells (lane 1 vs lane 2) was further reduced by adding 50 pg/ml of IL-1α (lane 3 with 10T1/2 cells). The mediated reduction of IFN-γ-production by UM containing recombinant IL-1α was comparable to that by CM. However, it is notable that further reduction of the IFN-γ level was not observed when increased concentrations of IL-1α (150 pg/micro l) were used, suggesting that Sq-1979 cells produce significant amount of IL-1α for this activity (lane 4 with 10T1/2 cells). Immunosuppressive function mediated by CM and IL-1α was not observed in the absence of 10T1/2 cells.
4. DISCUSSION
IL-1α is a unique cytokine possessing the dual function of binding to chromatin to regulate transcription as well as to cell surface receptors as a signaling molecule [19]. IL-1α is rarely secreted by living cells and in most cases is undetectable in body fluids [20]. Keratinocyte-derived IL-1α induces wound-induced papilloma formation [21]. In skin homeostasis, epidermal tissue regeneration is mainly regulated by keratinocyte -derived IL-1 signaling, which induces keratinocyte growth factor expression in skin fibroblasts [22].
Tumor cell derived IL-1α preferentially activates anti-tumor immunity. Membrane-associated IL-1α may thus serve as an adhesion molecule. Constitutive expression of membrane -associated (but not secreted) IL-1α by oncogene-transformed fibrosarcoma cells induced regression of tumors in mice by activating CD8+ T cells [23]. IL-1α production induced by cytokines/immunomodulators in T cell lymphoma cells also causes the stimulation of antitumor immune responses [24].
By contrast, protumoral (namely immunosuppressive) activity of IL-1α has been reported in tumor-stromal crosstalk. In OSCC tissues, IL-1α affects CAF to produce CCL7, CXCL1 and IL-8, which promote motility and invasion of tumor cells [25, 26]. Immunosuppressive function of IL-1α via CAF has been reported in the tumor milieu of melanomas harboring BRAF (V600E) oncogene, by which the expression of IL-1α is specifically activated [27]. In the prostatic carcinoma tissue, IL-1α upregulates TGF-β production from mesenchymal stem cells (MSCs), resulting in the potential suppression of immune cells and the promotion of tumor cells [28]. Our present results first demonstrate the immunosuppressive activity of IL-1α in OSCC cells. The reduced production of IFN-γ from antigen-stimulated spleen cells, attributable to direct contact with mesenchymal (10T1/2) cells was further reduced in the presence of IL-1α. Sq-1979 cells produced significant amount of IL-1α for this immunesuppression. The production of IL-1α is specifically elevated in Sq-1979 cells, however it is undetectable in L5-11 cells, one of the metastasized sub-clones of Sq1979 cells. The inability of L5-11 cells to enhance the immunosuppressive function of 10T1/2 cells has been confirmed [15]. We have already reported, the immunosuppressive efficacy of the OSCC milieu is developed in a stepwise manner depending on the stages of OSCCs [14, 16]. Therefore, in the early stage OSCC as represented by Sq-1979 cells, IL-1α from the tumor cells could functionally promote mesenchymal stromal cells as a unique effector exerting immune suppression via direct cell contact with activated T cells. These, however, do not induce any MDSCs in the tumor-bearing mice [16].
Our observations suggest that IL-1α may specifically contribute to the development of OSCC in early stages. In fact, IL-1 receptor antagonist (IL1RN) is significantly down regulated in early OSCCs compared to premalignant lesions and advanced OSCCs [29]. In head and neck SCC patients, significant correlation between IL-1α expression and development of distance metastasis has been reported [30]. The mechanism by which OSCCs differentially utilize immune modulatory aspects involving CAFs, MDSCs and several other factors is not fully understood. Among these aspects, the function of IL-1α seems to be pleiotropic depending on the tumor cell types. In melanoma tissues, immunosuppressive function of IL-1α is, in part, mediated by upregulation of programmed cell death ligand (PD-L) 1 and PD-L2 mRNAs in CAFs [27]. The inflammatory responses that result from periodontitis cause several miRNAs to exert control over all aspects of innate and adaptive immunity [31]. Furthermore, high level miR-197 expression is closely correlated with poor overall survival and reduced PD-L1 transcription in OSCC tissues [32]. However, in our observation, both PD-L1 and PD-L2 mRNAs were unchanged or undetectable in IL-1α-treated 10T1/2 cells, respectively (unpublished data).
CONCLUSION
In conclusion, IL-1α could be a promoter of motility, invasion and immune suppression in OSCC tissues. Further elucidation of the regulatory pathways involving IL-1α could identify important therapeutic targets in OSCC development.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Animal Ethics Committee of Asahi University (No. 17-038),
HUMAN AND ANIMAL RIGHTS
No humans were used for studies that are the basis of this research. All animal experiments were conducted according to American Veterinary Medical Association (AVMA) Guidelines for the Euthanasia of Animals: 2013 Edition
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data sets analyzed during the current study are available from the corresponding author on request.
FUNDING
This work was financially supported in part by the Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science KAKENHI Grant Number (JP17K11891).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publishers Website along with the published article.


