All published articles of this journal are available on ScienceDirect.
Peripheral Odontogenic Myxoma: Report of Two New Cases with a Critical Review of the Literature
Abstract
Introduction:
Odontogenic myxoma is a benign mesenchymal odontogenic tumor that is more frequently found in the mandibular molar region. The extra-osseous counterpart, called Peripheral Odontogenic Myxoma (POM), is an exceedingly rare entity and with very low aggressive potential.
Methods:
Clinical and radiographic findings are not specific, and difficulties may exist in differentiating histological findings of POM from other tumors with myxoid features, such as fibromas and oral focal mucinosis. Due to the fact that the presence of odontogenic epithelium is not required for the diagnosis, a myxoid lesion without odontogenic nests that develops in extraosseous position can be misdiagnosed with a soft tissue myxoma.
Case Reports:
We thereby present two cases of POM and conduct an extensive and critical review of the English literature, taking into consideration both certain and putative cases of POM.
1. INTRODUCTION
Odontogenic Myxoma (OM) is a benign mesenchymal odontogenic tumor included in the heterogeneous group of myxoid lesions. This tumor was identified for the first time in 1947 by Thoma and Goldman [1], while Stout described OM as a true mesenchymal neoplasm, in 1948 [2]. OM is characterized by stellate and spindle-shaped cells dispersed in an abundant myxoid extracellular matrix. When a greater amount of collagen is evident, the term “odontogenic myxofibroma” may be used [3]. OM is believed to originate from embryonic connective tissue associated with the tooth-bearing apparatus. The evidence for its odontogenic origin arises from several aspects, such as the almost exclusive location in the tooth-bearing areas of the jaws, the occasional association with missing or unerupted teeth, and the possible presence of odontogenic epithelium [4].
Peripheral Odontogenic Myxoma (POM) is considered the extra-osseous counterpart of OM. It is very rare and significantly less aggressive, compared to OM [5]. Furthermore, POM is a slow-growing tumor with a lower recurrence rate than its central counterpart, with no evidence of metastasis. The age range of affected patients is wide, with most of the cases found between the second and fourth decades of life, with an M:F ratio of 2:1 [3]. POM usually presents as painless and exophytic gingival mass, resulting in a slowly-growing swelling. The overlying epithelium is usually unaffected and no bony involvement is present [6]. POM appears macroscopically as a grey-white, mucoid mass with a smooth or multinodular external appearance and it usually is encapsulated or circumscribed [7]. POM may be difficult to differentiate microscopically from other tumors with myxoid features, risking being misdiagnosed with fibroma, peripheral odontogenic fibromas, or oral focal mucinosis. Diagnosis can be established only after histological examination of the lesion [5, 8-10]. Histological features of OM are almost identical to the dental papilla of a developing tooth or a normal dental follicle, and nests of inactive odontogenic epithelium may be present [3]. The aim of this report is to present two new cases of POM and to conduct an extensive and critical review of the English literature, taking into consideration both certain and putative cases of POM.
2. CASE REPORT
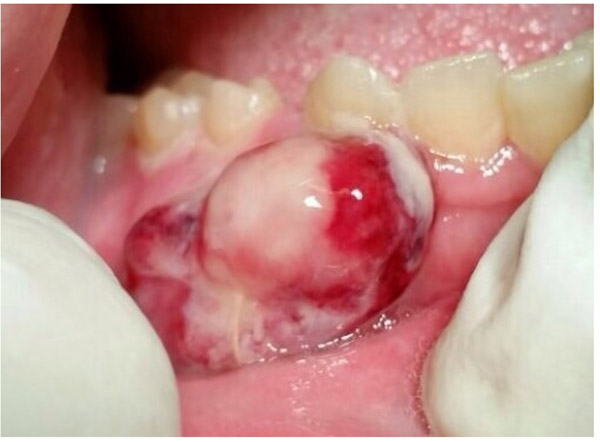
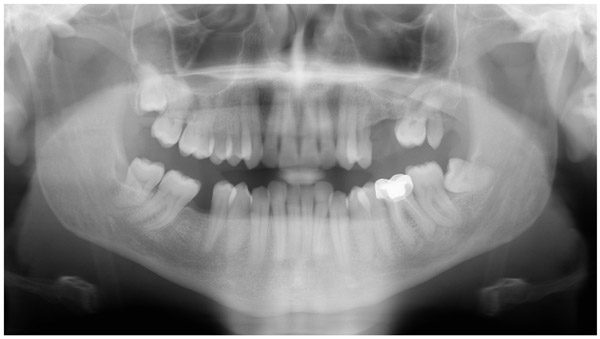
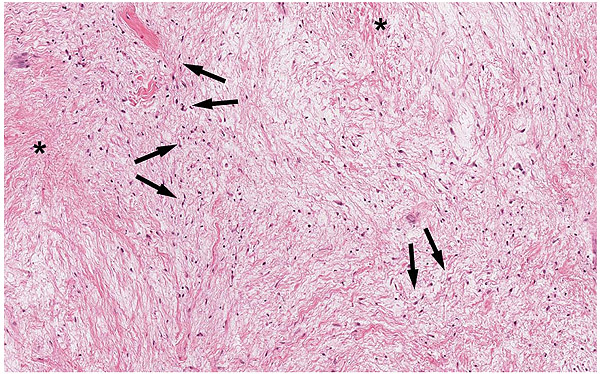
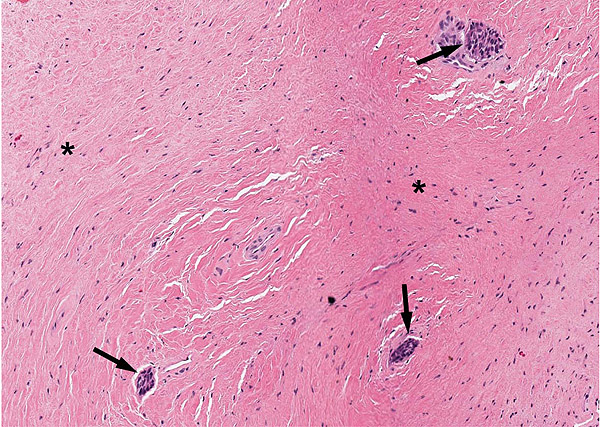
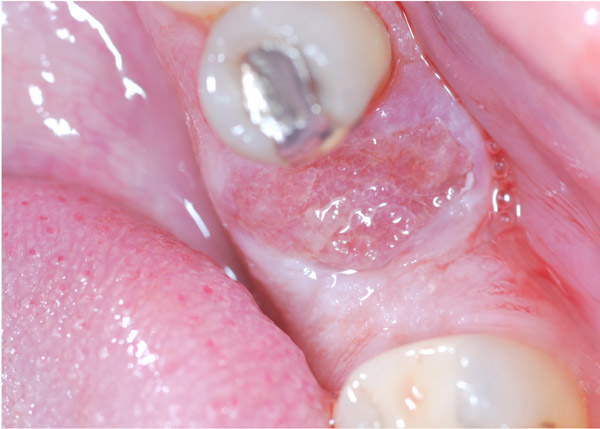
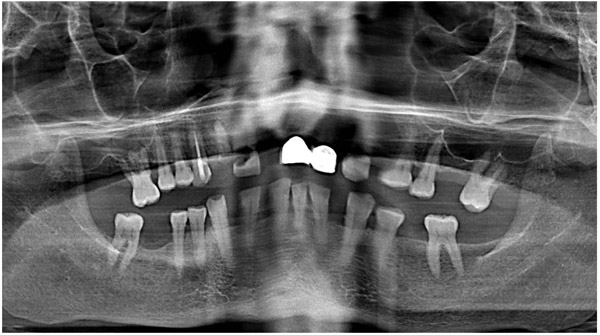
A 47-year-old male was referred to the Department of Maxillofacial Surgery, “Ospedali Riuniti” General Hospital, Ancona, with an asymptomatic large mass located on the right mandible mucosa, in the premolar region. This lesion had appeared 3 months previously and was beginning to interfere with mastication. Oral examination showed a tense-elastic mass measuring about 2-3 cm, with erythematous overlying mucosa (Fig. 1). Radiological examination showed the absence of erosion of the underlying bone (Fig. 2). Preoperative clinical diagnosis was oral fibroma; the lesion was completely excised and the tissue was sent for histological evaluation. Gross examination of the specimen revealed a withe nodule, with elastic consistency, measuring 2.5 x 2 cm. Microscopically, the lesion was characterized by stellate and spindle-shaped cells, embedded in an extensively vascularized fibro-myxoid extracellular matrix (Fig. 3). Scattered nests of inactive odontogenic epithelium surrounded by fibrous stroma were seen (Fig. 4). Neither atypia nor mitotic activity was seen. The mucosa is covered by epithelium-free of atypia, and the connective tissue showed chronic inflammatory infiltrate with hemosiderin iron deposits. Based upon these features, the diagnosis of POM was made. No evidence of recurrence was found after a 7-year follow-up.
The second patient was a 23 year-old male, referred to the Dental Clinic of Marche Polytechnic University, Ancona, with a 1-month history of progressive enlargement of an asymptomatic mass in the anterior right mandible mucosa. Oral examination showed a soft tissue mass measuring about 1 cm, with normal overlying mucosa, in inferior premolar region (Fig. 5). Radiological examination showed the absence of erosion of the underlying bone (Fig. 6). Preoperative clinical diagnosis was oral fibroma; the lesion was completely excised, and the tissue was sent for histological evaluation. Macroscopically, fragile fibrous fragments measuring 0.5 x 1 cm were appreciated. Microscopically, the lesion was similar to that in Case 1; a nest of inactive odontogenic epithelium surrounded by fibrous stroma was seen in Fig. (7). The morphological picture confirmed the diagnosis of peripheral odontogenic myxofibroma. No evidence of recurrence was found after a 9-year follow-up.
3. DISCUSSION
The term myxoma was first used by Virchow in 1863 to indicate a lesion characterized by stellate and spindle-shaped cells dispersed in an abundant myxoid extracellular matrix [11]. Actually, the term myxoma refers to a large and heterogeneous group of lesions with a different histologic origin that can affect any part of the body [12]. Within this group, in 1947, a new type of myxoid lesion has been identified. This tumor showed a typical involvement of the maxillary bones and was renamed OM by Thoma and Goldman [1]. The peculiarity of myxoid lesions is that a localization in bones other than the jaws is extremely rare, while OM is a relatively common odontogenic tumour [13, 14].

Since the 1930s, rare variants of odontogenic tumors have been identified, characterized by development in extraosseous position, for this reason, defined peripheral odontogenic tumors [15]. The histogenesis of this rare group of tumors is controversial: It could be derived from remnants of the dental lamina located over the periosteum, in the gingival tissues [16]. The possibility of identifying a peripheral variant of the OM has been debated since the 70s [17, 18], although years before, Pindborg highlighted the difficulty of identifying the histological origin of the so-called “Odontogenic Mesodermal Tumors” [19]. The idea about the possible existence of the POMs has been strengthened over time, and in the last 40 years, several cases of POM have been reported in the literature.
Based on that, only the recent cases of POM reported in the literature can be considered “Confirmed”, since the authors were aware of having identified a peripheral variant of OM. Therefore, all case reports before 1975 were considered “Bona fide” POM.
To date, a total of 30 cases of POM appeared in the literature, with two more additional cases presented in this article. The main features of POM are summarized in Table 1. The age of the patients ranges from 2 weeks to 71 years, with a peak in the third decade. The tumor shows a higher prevalence in females than males, and the most frequent localization of POM is the anterior maxilla.
| Sex | Females: 64.5% Males: 35.5% |
| Age | Mean: 32.4 ± 16.5 years (range: 2 weeks-71 years) Mean (Females): 32.1 ± 16.2 years Mean (Males): 32.9 ± 17.6 years |
| Location | Maxilla: 48,3% Mandible: 25.8% Palate: 16.1% Floor of mouth: 6.5% Other: 6.5% |
| Symptoms | Swelling: 67.8% Interference with oral function: 21.4% Facial asymmetry: 10.7% Asymptomatic: 7.1% Other symptoms: 14.3%. |
| Duration | Mean: 30.2 ± 78.9 months (Range: 2 weeks- 30 years) |
| Clinical presentation | Pedunculated mass: 31.2% Soft mass: 32.1% Hard mass: 25.0% Erythematous and/or ulcerated mucosa: 28.1% Tense-elastic mass: 15.6% Firm mass: 12.5% Multilobulated mass: 3.1% Granulomatous mass: 3.1% Generalized gingival hypertrophy: 3.1% Rapid growth: 3.1% |
| Size (cm) | Mean: 3.2 ± 2.7 cm (range 0.5-1.2 cm) |
| Histological diagnosis | Peripheral odontogenic myxoma: 80% Peripheral odontogenic myxofibroma: 20% |
| Odontogenic epithelium | Yes: 30% No/Unknown: 70% |
| Follow-up | Mean: 33 ± 38.5 months (range: 4-156) |
The clinical features of POM are not pathognomonic [20]. Swelling is the most common presenting manifestation, reported in 67.8% of cases, of which 4 cases were associated with other signs and symptoms. Only in 2 cases, the POM was asymptomatic and was discovered as an incidental finding. Other clinical manifestations as tooth mobility, bleeding, generalized gingival hyperplasia and sore throat, were rare. The duration of symptoms varies considerably, ranging from 2 weeks to 30 years. Macroscopically, frequent presentations were a pedunculated mass (31.2%), a soft (31.2%) or a hard mass (25.0%), an erythematous and/or ulcerated mucosa (28.1%). The maximum tumor size ranged from 0.5 to 12 cm, with a mean diameter of 3.2 ± 2.7 cm.
Adekeye confirmed the OM's origin from ectomesenchyme [21], however, POM histogenesis remains uncertain, so several theories have been developed regarding its origin. One hypothesis states that altered primitive fibroblast produces excess mucopolysaccharides. Other authors have suggested an origin derived from mesenchymal cells, such as dental papilla, dental follicle, or periodontal ligament, based on its histological similarity to the stellate reticulum of the developing tooth and its exclusive occurrence in close proximity to the tooth-bearing parts of the jaws [9]. However, further studies are necessary to clarify the origin.
The diagnosis of POM is essentially based on the same histological criteria of an OM, differentiating only on the extraosseous position. In fact, these tumors consist of hypocellular lesions composed of randomly oriented stellate, spindle-shaped and round cells with long, fine, anastomosing, pale or slightly eosinophilic cytoplasmic processes. The cells are evenly dispersed in an abundant myxoid ground substance that characteristically contains a minimal amount of collagen fibers. Binucleated cells, mild pleomorphism, and mitotic figures may occur and can mimic atypia. The late recognition of the existence of POM is suggested by the fact that a reference to the existence of this variant appeared only in the last WHO classification of odontogenic tumors [3]. Given the quite specific characteristics of the OM, the recognition of a peripheral variant implies a certain overlap with another lesion: the soft tissue myxoma. In fact, this myxoid lesion can affect any region of the body, including the head and neck region [22, 23]. Based on the characteristics of OM reported in WHO classification, microscopic nests of odontogenic epithelium may be found in about 5% of OM and are not required for establishing histological diagnosis since the use of clinical, radiological and histological data make the diagnosis of OM possible [3, 24]. In this literature review, 9 cases showed the presence of odontogenic epithelium. Diagnostic doubts occur in POM: in fact, a myxoid lesion without nests of odontogenic epithelium that develops in extraosseous position can be misdiagnosed with a soft tissue myxoma. Anatomical criteria are not always sufficient to distinguish these two tumors (e.g. myxomas originating from the cheek mucosa can be considered not of odontogenic origin, as well as other suspected odontogenic tumors [25]). On the other hand, considering the presence of odontogenic epithelium as an essential diagnostic criterion would exclude several cases that have been classified as POM. Furthermore, this would involve different diagnostic criteria between the central and peripheral variants of the same tumor. Based on the analysis of literature data, it seems that the percentage of POM with odontogenic epithelium is superior to the central counterpart. This difference could be a bias, or it could even further justify the need for a different classification criterion between POM and OM.
If left untreated, POMs have unlimited growth potential; a phenomenon which distinguishes POM from reactive non-neoplastic polypoid gingival growths. The treatment of choice is surgical excision, accompanied by extraction of involved teeth in 4 cases. POMs without bone destruction are treated with simple surgical excision, while those with bone destruction require excision and marginal curettage. 24 cases underwent regular postoperative control, with a follow-up period ranging from 4 months to 13 years. Some authors suggested that POM seems to have a much lower recurrence rate than the central counterpart. Therefore, a carefully planned conservative enucleation or semi-radical approach is justified, while other procedures are not recommended [6]. Overtreatment of POMs should be avoided in lesions close to vital structures and in pediatric patients, as it may affect the alignment and eruption of the teeth [9]. Recurrence is usually due to incomplete removal of the tumor and lack of capsule. Therefore, the follow-up period is clearly necessary to rule out intraosseous extension and recurrences. POMs are less aggressive than their central counterparts and only one case reported in the literature have recurred [26]. However, it is recommended to use the same follow-up protocol of OM, consisting in a close monitoring for at least the first 2 years after surgery; a period during which the neoplasm is most likely to recur [27]. Analysis of the literature data, including confirmed and “bona fide” cases, is recapitulated in Table 2.
| Author (Year) [Ref] | Age/Sex | Symptoms (Duration) | Clinical Presentation | Site (Teeth) | Size (cm) | Od. Ep. | Follow-up |
|---|---|---|---|---|---|---|---|
| *Daniels et al (1908) [28] | 27/F | Swelling (9 m) | Hard and firm mass, rapid growth | Post right mand. (4.4-4.5) | / | / | / |
| *Tholen et al (1936) [29] | / | / | / | Palate | / | / | / |
| *Babbit et al (1937) [30] | 14/F | Swelling, sore throat (3 m) | Soft mass | Palate | 6-7 | / | 6 m |
| *Sealey et al (1948) [31] | 51/F | Swelling (30 y) | Hard pedunculated mass | Palate | 3 x 2 | / | / |
| *Salama et al (1951) [32] | 45/M | Swelling (11 y) | Hard pedunculated mass | Palatal post max. (2.4-2.6) | / | / | / |
| *Pradhan et al (1972) [33] | 32/F | Interference oral function | Pedunculated mass | Palatal ant. max. (1.1-2.1) | 1.8 x 1.8 | / | 12 m |
| Tahsinoglu et al (1975) [34] | 34/F | Swelling | Hard mass | Ant left max. (2.1-2.2) | 0.5 x 0.5 | / | 4 m |
| Schneider et al (1975) [26] | 26/F | Swelling (5 y) | Hard mass | Ant right max. (1.2-1.4) | / | / | 48 m |
| Matsumara et al (1977) [35] | 2w/M | Interference oral function (2 w) | Soft pedunculated mass | Floor of mouth | 2.8 x 2.3 x 2 | / | 12 m |
| Swart et al (1977) [36] | 56/F | Swelling (3 w) | Soft mass, ulcerated mucosa | Floor of mouth | 4 | / | 36 m |
| Elzay et al (1978) [37] | 35/F | Swelling (4 m) | / | Palate | 3 x 2 x 1.3 | / | 156 m |
| 28/M | Swelling | Soft mass | Post left mand. | 2 x 2 | / | 36 m | |
| Rapidis et al (1983) [38] | 25/F | Asymptomatic (2 y) | Tense-elastic mass, normal mucosa | Palatal post. max (1.4-1.5) | 1.2 x 1 x 0.8 | / | 18 m |
| Nakano et al (1985) [39] | 71/F | Swelling, pain, bleeding, tooth mobility, facial asymmetry (1 m) | Tense-elastic and pedunculated mass, erythematosus mucosa | Palate, tuber (1.7-1,8) | 5 x 5 x 3.5 | / | 8 m |
| Siar et al (1986) [40] | 50/M | / | / | Palate | / | / | / |
| Gűnhan et al (1991) [41] | 27/F | Swelling (1 y) | / | Ant. left max. (2.2-2.3) | 1.3 x 1.3 | / | 48 m |
| Quintal et al (1994) [42] | 15/M | Swelling (4 m) | Multi-lobulated mass | Palate | 3 x 3 x 2 | / | 12 m |
| Shimoyama et al (2000) [43] | 51/M | Swelling, interference oral function (1 y) | Granulomatous and pedunculated mass, erythematosus mucosa | Ant left mand. (3.2) | 3 x 2.8 | Yes | / |
| Chang et al (2001) [44] | 37/M | / | Hard and firm mass | Post right mand. (4.8) | 1.5 x 1.2 x 1 | / | 8 m |
| Ramaraj et al (2003) [45] | 35/F | Facial asymmetry | Hard, firm pedunculated mass | Left max. (2.3-2.8) | 7 x 5 x 4 | / | 12 m |
| Perrotti et al (2006) [7] | 42/M | Asymptomatic | Tense-elastic mass, normal mucosa | Ant left max. (2.2) | 0.8 x 0.8 | Yes | 48 m |
| Aytac-Yazicioglu et al (2008) [46] | 38/F | Swelling (1 y) | Tense-elastic mass, erythematous mucosa | Post. right max. (1.4-1.6) | 3 x 2 | / | / |
| Nazarov et al (2008) [47] | 16/F | Swelling (1 y) | Tense-elastic mass, erythematous mucosa | Ant left max. (2.2) | 1 x 1 | Yes | 12 m |
| Raubenheimer et al (2012) [6] | 53/F | Interference oral function | Soft pedunculated mass | Ant left max. (2.1-2.2) | 12 x 6 x 5 | Yes | 84 m |
| 38/F | Interference oral function | Mass with erythematous and ulcerated mucosa | Ant mand (3.3-4.3) | 8 x 6 x 4 | Yes | / | |
| Jain et al (2013) [4] | 41/M | Swelling (6 m) | Hard mass | Ant left max. (2.1-2.2) | 2 x 1 | / | 6 m |
| Kapoor et al (2015) [27] | 11/M | Swelling, pain, facial asymmetry (1 y) | Soft mass, normal mucosa | Left infratemporal fossa | 2 x 2 x 2 | / | 24 m |
| Tasnime et al (2016) [21] | 14/F | Swelling (3 m) | Soft pedunculated mass, erythematous mucosa | Ant right max. (1.2-1.3) | 2 x 2 | Yes | 9 m |
| Bhoyar et al (2016) [48] | 8/F | Gingival hyperplasia (4 y) | Generalized gingival hypertrophy, hard mass | All gingiva | / | Yes | / |
| Kanitkar et al (2017) [9] | 12/F | Swelling (3 m) | Firm mass, normal mucosa | Ant left mand. (3.2-3.3) | 1.5 x 1 | / | 24 m |
| Bajpai et al (2017) [5] | 12/F | Swelling (3 m) | Soft and hard mass, erythematous mucosa | Ant. left max. (2.1-2.3) | 2 x 1 | Yes | 12 m |
| Present cases | 47/M | Interference oral function (3 m) | Tense-elastic mass, erythematous mucosa |
Ant. right mand. | 2.5 x 2 | Yes | 84 m |
| 23/M | Swelling (1 m) | Soft mass | Ant right mand | 1 x 0.5 | Yes | 108 m |
CONCLUSION
In conclusion, very few case reports of POMs are available in the literature. Usually, this tumor presents as an asymptomatic, exophytic gingival mass without bony involvement. Clinically and histologically, POMs resemble many other soft tissue lesions, so they must be diagnosed by histological examination. POM is less aggressive compared to its central counterpart, that must be treated by surgical excision. It shows evidence of recurrence only in one case; however, a follow-up period is recommended.
List of abbreviations
| OM | = Odontogenic Myxoma |
| POM | = Peripheral Odontogenic Myxoma |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Written informed consent has been obtained from all patients.
CONFLICT OF INTEREST.
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Marco Mascitti and Andrea Santarelli described the clinical cases. Marco Mascitti and Filippo Pirani conceived the literature review. Lucrezia Togni and Filippo Pirani wrote the manuscript with support from Marco Mascitti. Corrado Rubini and Lucrezia Togni performed histopathological analysis and revisited critically the manuscript. All authors discussed and approved the final version of the manuscript to be published.


