Effect of Denture-Related Stomatitis Fluconazole Treatment on Oral Candida albicans Susceptibility Profile and Genotypic Variability
Abstract
Denture-related stomatitis (DRS) is the most common condition affecting removable-denture wearers, and Candida albicans the most frequent pathogenic agent. Systemic antifungal treatment is indicated but recurrences are frequent. The aim of this study was to characterize the oral load, fluconazole susceptibility profile and genotypic variability of oral C. albicans isolates from patients with DRS before (T0), immediately after fluconazole treatment (Tat) and after 6-months follow-up (T6m). Eighteen patients presenting DRS and treated with fluconazole were followed at the Faculty of Dentistry of Oporto University. Seventy C. albicans isolates were obtained and identified using standard cultural and biochemical multi-testing. Fluconazole susceptibility was tested by E-test®. Microsatellite-primed PCR was performed to assess the genotypic variability of C. albicans isolates. The patients’ mean age was 58.0±3.2 years, and 55.6%/44.4% had total/partial dentures. Before treatment, 22.2%, 44.4% and 33.3% of the patients presented DRS type I, II or III, respectively. Fluconazole treatment healed or improved DRS in 77.8% of the patients, accompanied by an 83.5% reduction in oral C. albicans load. However, after 6-months, oral C. albicans load increased significantly and DRS severity was similar to the one observed before treatment. Moreover, the prevalence of patients presenting fluconazole resistant isolates of C. albicans increased significantly throughout the study: T0-5.6%, Tat-10.0% and T6m-42.9%. A change in the genotypic variability of C. albicans isolates was also verified, being mostly associated to fluconazole susceptibility profile change. In conclusion, fluconazole presents a good short-term DRS treatment efficiency, but may be associated to a long-term emergence of C. albicans fluconazole resistance.
INTRODUCTION
Denture-related stomatitis (DRS) is defined as an inflammatory process of the oral mucosa underlying a removable, partial or total, dental prosthesis. In patients with removable prostheses, the mean prevalence of DRS is 50% [1, 2]. Aetiological factors in DRS include the trauma caused by an ill-fitting denture, lack of oral and prosthesis hygiene and a favourable environment for the proliferation of microorganisms [3]. Although bacterial infection, mechanical irritation, and allergic reaction have been proposed as possible causes of DRS, infection with Candida species is often implicated [1, 2, 4].
Candida spp. are oral commensals present in up to 90% of healthy persons and Candida albicans (C. albicans) is the most frequent colonizer fungi [5-7]. Previous studies from our group showed that DRS is clearly associated with oral infection by yeasts, mainly C. albicans [1], although the same genotype may be found both in DRS patients and in healthy controls [8]. Although DRS is not a particularly severe pathology, it may be a portal of entry for further infections mainly in susceptible patients [1, 2].
There is clear evidence that the management of Candida-associated denture stomatitis is complex due to its multifactorial aetiology [9-1]. Current treatment includes control of denture plaque, and, with patient compliance, some rest periods of denture wearing in addition to the use of antimicrobial agents [12, 13]. Topical antifungal drugs, such as nystatin, amphotericin B, and miconazole have proved to be effective in DRS improvement [14-16]. However, recurrence rates are high and treatment regimens tend to be extended [17, 18]. The systemic triazoles, such as fluconazole, are currently the first therapeutic choice for the treatment of this infection [19, 20], although progressive recolonization of the palate and the denture fitting surface by yeast, together with a high rate of clinical relapse and recurrence after therapy, have also been reported [14, 21]. So, the aim of this study was to characterize during a 6-months period the oral load, fluconazole susceptibility profile and genotypic variability of oral C. albicans isolates from DRS patients treated with fluconazole therapy.
MATERIAL AND METHODS
Study Design and Patient Selection
Twenty-one patients wearing polymethylmethacrylate maxillary removable prosthesis, with clinical diagnosis of DRS, followed at the outpatient clinic of the Faculty of Dentistry of Oporto University were randomly selected. Patients were assessed clinically and saliva samples were collected for microbiological analysis at the first clinical evaluation and diagnosis (T0), after fluconazole treatment (Tat) and after a 6-month follow-up period (T6m). Fluconazole was prescribed systemically (Fluconazol 50mg, Diflucan® Pfizer) for 15 days to all patients. After this period, systemic fluconazole was prescribed for further 15 days only to patients who did not improve clinically from DRS.
Ethics Committee of the Faculty of Dentistry of Oporto University reviewed and approved this study. All participants were recruited voluntarily after receiving detailed information on the study protocol. Free and written informed consent was obtained from all participants, according to the Helsinki Declaration.
Clinical Oral Evaluation
To identify and characterize the different DRS presentations, the Newton classification was used: DRS type I (localized inflammation or hyperaemia points), DRS type II (diffuse erythema) and DRS type III (palate papillary hyperplasia) [22].
Denture hygiene was assessed by modification of the Tarbet index [1, 4, 23].The patients were classified according to the total denture surface covered with microbial plaque evaluated by a dental plaque disclosing solution (Dento-plaque®, Pierre-Fabre Dermo-Cosmetique) in the following groups: poor denture hygiene, >76%; insufficient denture hygiene, 26 to 75%, and good denture hygiene, < 25%.
Additionally, before the oral clinical evaluation, unstimulated whole saliva was collected in a sterile container at least 2h after eating, tooth brushing, mouth washing or smoking.
Candida albicans Isolation and Quantification
Saliva samples from all patients were serially diluted with 0.9% sterile NaCl solution until 10-1 and immediately plated in triplicate in a selective and differential culture medium, CHROMagar CandidaTM® (Becton Dickinson, Sparks, USA). Afterwards, plates were incubated aerobically for 48h at 37°C. The total number of green-pigmented colonies (Candida albicans) were counted and isolated. The lower limit of detection was 1CFU/mL. C. albicans isolates were identified based on germ-tube formation, chlamydospore production and carbohydrate assimilation using the ID 32C system (BioMerieux, Marcy l'Etoile, France). The yeasts identified as C. albicans were also screened for their ability to grow on Sabouraud dextrose agar at 45ºC for 48h and xylose assimilation, to distinguish from C. dubliniensis [24]. Several colonies of each different phenotype were selected and kept frozen at -70ºC, in sterile water with 30% glycerol. Prior to testing, the yeasts were subcultured in Sabouraud dextrose agar (BioMerieux, Marcy l'Etoile, France) and incubated at 37ºC for two days. A total of 70 C.albicans isolates were obtained at T0, Tat and T6m from all patients. C. dubliniensis FFUL 21 and Candidaalbicans ATCC 90028 were used as internal controls for the identification tests.
Antifungal Susceptibility Testing
Susceptibility testing to fluconazole was performed by Etest® (BioMerieux, Marcy l'Etoile, France), according to the manufacturer’s instructions. C. albicans isolates were classified accordingly to the proposed in vitro breakpoints to fluconazole: susceptible, minimal inhibitory concentration (MIC) ≤8mg/L; susceptible-dose dependent (S-DD), MIC between 16 to 32mg/L; and resistant, MIC ≥64mg/L.
Microsatellite-Primed PCR
For microsatellite-primed PCR genomic DNA was extracted in accordance with Treco [25]. Nucleic acid purity and concentration were assessed by spectroscopy at 260 and 280nm. A single repeat sequence of the phage M13, 5’-GAG GGT GGC GGT TCT-3’ was used in the microsatellite-primed PCR experiments, because the array of DNA fragments presented high intensity bands and identical patterns of DNA fragments were detected with replicate samples, showing to be the best primer tested [26]. Synthetic oligodeoxyribonucleotide molecules were prepared by Oligo-Express (Paris, France). Amplification reactions were performed in volumes of 50mL containing 25ng template DNA, 10mmol/L Tris-HCl (pH 8.3), 50mmol/L KCl, 4mmol/L MgCl2, 200mmol/L of each dNTPs, 1 mmol/L of primer, and 2.5U Taq DNA polymerase. Except for dNTPs, obtained from Amersham Pharmacia Biotech (Uppsala, Sweden), all PCR reagents were purchased from Perkin Elmer Corp., Applied Biosystems (New Jersey, USA). The samples were amplified in a Biometra T1 termocycler (Göttingen, Germany), and the thermal cycling parameters were an initial denaturation at 95ºC for 3min, followed by 35 cycles of denaturation at 95ºC for 45s, annealing at 46ºC for 40s, and extension at 72ºC for 30s with final extension at 72ºC for 5 minutes. PCR products were analysed by electrophoresis with agarose gel stained with ethidium bromide. DNA Ladder and pBR322 DNA-BstNI digest (New England Biolabs, Beverly, Mass.) were used as molecular size standards. To ensure the reproducibility of the results, reference strains were systematically run as controls together with negative controls (no DNA). The profiles for each isolate were compared visually with identical band patterns deemed to represent the same genotype.
Statistical Analysis
The statistical analysis was performed using IBM® SPSS® version 19.0 (Statistical Package for Social Sciences). The categorical variables were described through absolute and relative frequencies (%) and analysed by Chi-square independence test. Continuous variables were described using mean ± standard error (SEM) and analysed by Anova followed by students’ t-test. Statistical significance was assumed when the p values were less than 0.05.
RESULTS
The first clinical evaluation and diagnosis (T0) was performed to 21 patients presenting a mean age of 57.6 years with a standard error of 3.1 years; 28.6% were males and 71.4% were females. Three patients (14.3%) did not conclude the study due to different reasons – death, hospitalization and unknown whereabouts - and were excluded thus from the analysis. The characterization of the group of participants followed during the 6-month period is shown in Table 1 (n=18). All patients had dentures of polymethylmethacrylate resin. At the time of the first clinical evaluation most of the patients presented DRS type II and insufficient denture hygiene (Table 1).
Characterization of patients with denture-related stomatitis (DRS) regarding age, denture type and denture hygiene at the time of first diagnosis (T0).
| DRS patients (n=18) |
|
|---|---|
| Age, years | 58.0±3.2 |
| Gender, male / female | 33.3% / 66.6% |
| Total / partial denture | 55.6% / 44.4% |
| DRS type I / II / III | 22.2% / 44.4% / 33.3% |
| Poor / insufficient / good denture hygiene | 16.7% / 66.7% / 16.7% |
Values are mean±sem or prevalence (%).
Fluconazole response of the followed up patients.
| Fluconazole treatment period |
Healed | Improved | Not improved |
|---|---|---|---|
| 15 days | 27.8% | 27.8% | 44.4%* |
| Plus 15 days | 11.1 % | 11.1% | 22.2% |
* These patients did further 15 days of fluconazole therapy.
After 15 days of fluconazole treatment, 55.6% of the patients healed or improved DRS (Table 2). The remaining 44.4% presented DRS type I or II, evenly. These patients were prescribed further 15 days of fluconazole treatment. Half of the patients with 30 days of fluconazole treatment healed or improved DRS. In total, fluconazole therapy healed or improved DRS in 77.8% of the patients, regardless of the duration of the treatment. After the fluconazole treatment, 38.9% of the patients presented no clinical evidence of DRS, 44.4% presented DRS type I, 16.7% presented DRS type II, and no patient presented DRS type III (Fig. 1). After the 6-months follow up period, the DRS worsened in most of the evaluated patients and DRS severity was similar to that observed before treatment (Fig. 1). The prevalence of DRS type changed significantly throughout the study (Fig. 1, p=0.001).
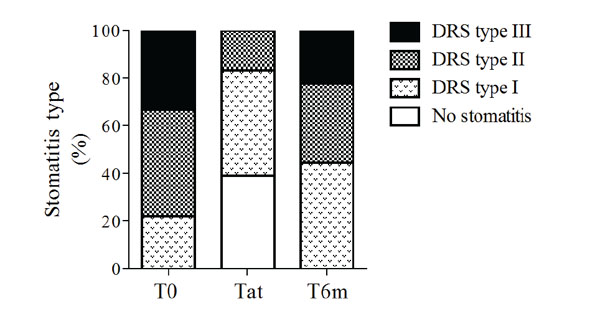
Denture-related stomatitis (DRS) type at the time of stomatitis diagnosis (T0), after treatment (Tat) and 6-months after diagnosis (T6m). Bars represent prevalence.
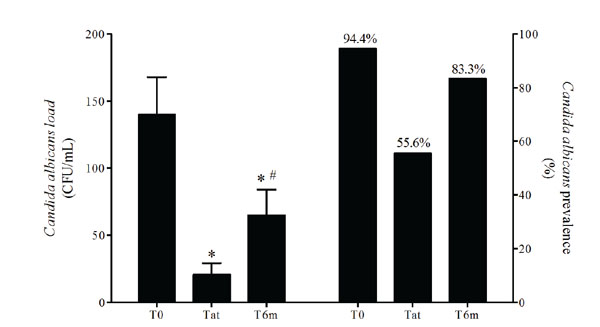
Oral Candida albicans load and prevalence at the time of stomatitis diagnosis (T0), after treatment (Tat) and 6-months after diagnosis (T6m). Left bars represent means and error bars represent SD. p values were calculated using Anova (p=0.00038) followed by students’ ttest. *p<0.05, significantly different from T0; #p<0.05, significantly different from Tat. Right bars represent prevalence.
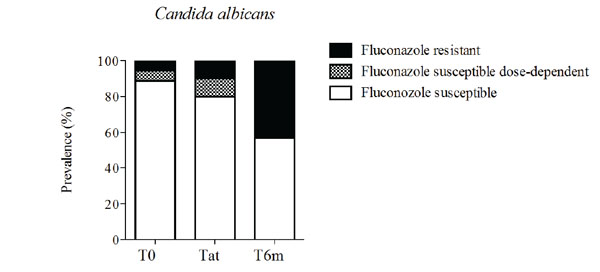
Prevalence of fluconazole susceptibility profile of Candida albicans isolates at the time of stomatitis diagnosis (T0), after treatment (Tat) and 6-months after diagnosis (T6m). Bars represent prevalence.
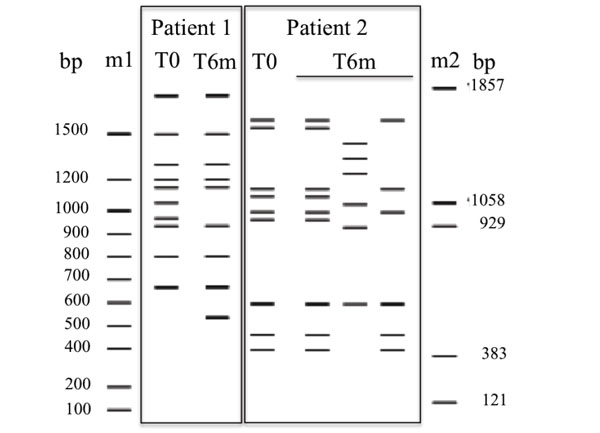
Genotypic variability of Candida albicans isolates from two different patients studied by MSP-PCR with the primer M13 at the time of DRS diagnosis (T0), and 6-months after diagnosis (T6m). Molecular size markers used were DNA Ladder (m1) and pBR322 DNA-BstNI digest (m2).
At T0, the oral C. albicans prevalence in the evaluated patients was 94.4% and the mean load was 139.9±27.9CFU/mL of saliva. Fluconazole treatment reduced oral C. albicans prevalence and load in saliva, but at T6m the C. albicans prevalence increased, as did the C. albicans load (Fig. 2). The oral C. albicans prevalence profile differed significantly throughout the study (p=0.015).
At T0, the fluconazole susceptible dose-dependent and resistant isolates of C. albicans represented 5.6% each, whereas the susceptible isolates represented 88.9% (Fig. 3). After the 6-month follow-up period the C. albicans resistant isolates represented 42.9%, whereas the susceptible isolates represented 57.1% (Fig. 3).
The analysis of genotypic variability of C. albicans isolates, using primer M13, generated profiles with 6 to 14 bands varying between 400 and 1,800bp. From all evaluated patients that presented C. albicans counts in more than in one evaluation point, 42.9% showed a change in genotypic variability of C. albicans isolates throughout the study. Two examples of C. albicans profile change from T0 to T6m are shown in Fig. (4). Patient 1 presents two different genetic profiles, whereas patient 2 presents 3 different C. albicans genetic profiles. Identical profiles were obtained for all replicate samples, confirming the high reproducibility of this method. From this group of patients presenting C. albicans genotypic variability of M13 primer throughout the study, 83.3% had at least one C. albicans isolate resistant to fluconazole.
DISCUSSION
The present work intended to evaluate the effect of fluconazole treatment on short-term and long-term clinical development of DRS and C. albicans oral colonization profile.
Several factors may contribute to the onset and worsening of DRS, namely poor denture hygiene, continuous wearing of removable dentures, accumulation of denture plaque, bacterial and yeast contamination of denture surface and mucosal trauma due to poor-fitting dentures. In the present study each patient was his own control, since patients were evaluated before and after fluconazole treatment. Changes were not observed throughout the study in denture substitution or adjustment, denture hygiene promotion and denture daily wearing period, factors that could influence DRS progression besides fluconazole therapy.
There is a large body of evidence indicating that Candida is able to adhere to acrylic resin dentures, namely to polymethylmethacrylate – PMMA, forming a biofilm [27, 28]. As expected and in agreement with previous reports, denture wearer patients presented high rates of C. albicans oral colonization (94.4%) at the time of the first clinical evaluation (T0) [29-31]. This denture biofilm provides a source of microorganisms that is continuously exposed to the oral mucosa. This is the first step that may lead to the development of the infectious process and that may ultimately result in varying degrees of denture stomatitis of the adjacent mucosa [29, 32].
As expected, fluconazole treatment significantly reduced the oral C. albicans colonization and greatly improved the severity of DRS in the studied population [29, 33, 34]. However, these effects were observed only immediately after treatment (Tat). After the 6-month follow-up period (T6m), either oral C. albicans colonization or DRS severity profiles were similar to the ones observed before fluconazole treatment, revealing a good short-term but not a good long-term fluconazole DRS treatment efficiency. This fact probably reflects recontamination by residual yeasts that are present on the denture surfaces [29].
Additionally, the C. albicans oral population appear to change over time due to the increase of C. albicans isolates resistant to fluconazole 6-months after therapy. This hypothesis was reinforced by the high rate of genetic variability found in C. albicans isolates within each patient when isolates collected before and 6-months after fluconazole treatment were compared. Notwithstanding, no specific C. albicans resistant genes were analysed, such as the efflux pumps [35]. Increasing scientific literature addresses this phenomenon of antimicrobial resistance induced by therapeutic treatment regarding non-oral infections [36]. In oral cavity, antimicrobial resistance is still scarce; however, dental medical doctors should be aware of its increasing importance and future implication. Despite the good short-term fluconazole efficiency in the treatment of DRS, the fluconazole-generalized prescription should be avoided to reduce the emergence of resistant strains of C. albicans and the increasing episodes of DRS recurrences. Therefore, other therapeutic or prevention strategies should be implemented before fluconazole is recommended, namely denture disinfection together with denture hygiene education and surveillance, denture adjustment or replacement [20]. In addition, new developments related to denture materials are focusing on means to reduce development of adherent biofilms. These developments may be valuable in reducing yeast colonization, and could lead to reductions in denture stomatitis with appropriate denture hygiene [29, 37].
Nevertheless, some clinical scenarios may require fluconazole use, namely in DRS in immunosuppressed patients at a risk of a systemic infection or patients with persistent severe symptomatologic DRS [20].
CONCLUSION
In conclusion, fluconazole presents a good short-term, but not a good long-term, DRS treatment efficiency. Also fluconazole treatment of DRS may be associated to a long-term emergence of oral C. albicans fluconazole resistance. Other therapeutic or prevention strategies should be implemented before fluconazole is prescribed, namely denture disinfection, together with denture hygiene education and surveillance, as well as denture adjustment or replacement.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


