Oral Cavity Squamous Cell Carcinoma - Characteristics and Survival in Aboriginal and Non-Aboriginal Western Australians
Abstract
Background:
Squamous cell carcinoma (SCC) is the most common type of malignancy affecting the oral cavity. While exposures to main risk factors for oral SCC such as smoking and alcohol use are higher amongst the Aboriginal people, little is known about oral cancer in this population. This study aimed to describe characteristics and survival of oral SCC in Aboriginal and non-Aboriginal Western Australians.
Methods:
All primary oral SCC cases reported to the Western Australian Cancer Registry (WACR) between 1990 and 1999 were analysed with respect to person characteristics including: date of birth, sex and indigenous status; and disease characteristics including: date of biopsy, disease stage and site as well as date of recurrence and date of death. Exclusion criteria included diagnosis not based on incisional or excisional biopsy, diagnosis other than oral SCC or a history of another malignant neoplasm.
Results:
Aboriginal individuals were more likely to reside in rural areas. No statistically significant differences in oral SCC characteristics and survival were noted between Aboriginal and non-Aboriginal Western Australians.
Conclusion:
This study provides new information on person and disease characteristics of Aboriginal Western Australians diagnosed with oral SCC.
INTRODUCTION
Squamous cell carcinoma (SCC) is the most common type of malignancy affecting the oral cavity and the oropharynx, accounting for over 90% of cases [1]. Australia wide about 2,500 new cases of oral and oropharyngeal cancer (ICD 10 codes: C00-C06 and C090-C10) are reported annually [2]. Treatment modalities of early cancers include surgery or radiation therapy [3]. Advanced disease requires multimodality approach in the form of chemo-radiation therapy or surgery and radiation therapy / chemo-radiation therapy [3]. Despite advances in oral cancer treatment, survival continues to remain poor, which is largely due to late disease presentation [4]. Treatment failures include loco-regional recurrences, metastatic disease and second primaries [5]. Tumour grade, site, size and nodal involvement constitute important prognostic indicators [5]. Fortunately for the majority of individuals, oral cancer is a potentially preventable disease through lifestyle modifications particularly centred around cessation of tobacco and moderation of alcohol use [6]. Solar irradiation, micronutrient deficiency and infections with the human papilloma virus also constitute important risk factors for oral and oropharyngeal SCC [2, 7].
While imperfect, the Tumour, Node, Metastasis (TNM) Classification of Malignant Tumours system, based on the anatomical extent of disease, is widely used to classify oral SCCs [8, 9]. Within this classification system four disease stages are identified with all diseases falling within the particular disease stage exhibiting similar survival rates [8]. Generally, stage I and II disease is localized to the organ of origin; stage III disease is associated with local spread, particularly to the regional lymph nodes; with stage IV disease being the most advanced, where tumour spread may include presence of distant metastases [8].
Literature regarding oral cancer, disease specific cumulative survival and associated factors affecting survival of Aboriginal Australians with oral SCC is virtually non-existent [10]. Reasons for this may include: the Aboriginal population comprising only a small minority population; inadequate identification of indigenous people in administrative data collections; and cancer in general being considered a lesser problem for Aboriginal populations, in view of their significantly shorter life expectancy [10, 11].
It is widely accepted that exposures to the main risk factors for oral cancer such as smoking and alcohol use are higher in the Aboriginal population [12]. The remote residence of most Aboriginal individuals often limits access to appropriate medical services [13]. Difficulties in arranging transport and accommodation, the culturally foreign hospital environment and financial barriers constitute significant obstacles and are in part responsible for the significantly higher rates of hospital appointment cancellation and non-attendance as well as frequent hospital discharge against medical advice [13]. All these factors potentially impact on oral cancer incidence, disease presentation and survival of Aboriginal individuals.
The overall objective of this study was to describe Aboriginal oral cancer and investigate the 5-year survival and recurrence of oral SCC (restricted to ICD10 codes: C01-C06) in Aboriginal compared to non-Aboriginal population data using data available from the Western Australian Cancer Registry (WACR).
The specific aims were to:
- Describe the person and disease characteristics of individuals diagnosed with oral SCC in Aboriginal compared to non-Aboriginal population.
- Describe simple survival of individuals diagnosed with oral SCC in Aboriginal compared to non-Aboriginal population.
METHODS
This was a retrospective, observational study and an extension of our previously published work [14]. We compared person and disease characteristics, time to recurrence and survival of individuals diagnosed with oral SCC in Aboriginal and non-Aboriginal populations. The study was an investigation of Aboriginal and non-Aboriginal population data from the WACR, comprising all primary oral SCC cases with a diagnosis made over a ten year period between 1st of January 1990 and 31st of December 1999. Collection and use of data by the WACR are governed by the Health (Western Australian Cancer Register) Regulations 2011. According to the regulations, all cases of in situ neoplasms, invasive malignancies (except skin melanomas, SCCs and basal cell carcinomas) and benign central nervous system tumours must be reported to the WACR [15].
Data collected included: date of birth, sex, indigenous status, date of biopsy, disease stage (TNM classification), disease site (ICD10 codes: C01-C06), date of tumour recurrence and date of death. Where the WACR data were incomplete, individual’s hospital medical records were also examined for the relevant information. Data pertaining to the disease stage were obtained from hospital records alone as these data were not collected by the WACR. For practical reasons extraction of medical records for data collection was limited to the Western Australia’s three main pubic hospitals. For confidentiality reasons, details of the hospitals are not disclosed. Exclusion criteria included diagnosis not based on incisional or excisional biopsy; diagnosis other than oral SCC; and a history of any other malignant neoplasm diagnosed either prior to, simultaneously with or within the first five years following diagnosis of oral SCC.
Statistical Analysis System (SAS) 9.2 software was used for the descriptive and survival analyses. Kaplan Meier curves and Log-rank tests were used to compare the five-year survival of Aboriginal and non-Aboriginal oral SCC cases. Outcomes measured included:
- Time (in years) from initial biopsy of oral SCC to carcinoma recurrence. Recurrences included local, regional and distant metastases.
- Time (in years) from initial biopsy of oral SCC to carcinoma related death.
- Time (in years) from initial biopsy of oral SCC to death from any cause.
Approvals for the study were obtained from the Human Research Ethics Committees of University of Western Australia, all participating hospitals, the Western Australian Aboriginal Health Information and Ethics Committee and the Department of Health WA.
RESULTS
Between the 1st of January 1990 and the 31st of December 1999, 683 WACR records of oral SCC biopsy were identified for 656 individuals. One hundred and ninety seven records were excluded from the 683 WACR records, as these cases did not satisfy our eligibility criteria (Table 1). Examination of hospital records led to exclusion of another 62 records due to the missing or illegible nature of the records. The person characteristics of these 62 excluded cases were comparable to those included for analysis (64% male, mean age: 63 years, 74% metropolitan, 5% Aboriginal). Table 1 summarises the particulars of all cases excluded. Four hundred and twenty four individuals satisfied our inclusion criteria. Of these, only 16 individuals were identified as Aboriginal (4%).
Summary of reasons for exclusion of records.
| Reasons for Exclusion | No of Records Excluded |
|---|---|
| FNA based diagnosis | 17 |
| Prior Cancer | 88 |
| Prior and simultaneous cancer | 3 |
| Prior and later cancer | 21 |
| Simultaneous multiple cancers | 4 |
| Simultaneous and subsequent cancers | 3 |
| Later cancer | 58 |
| Carcinoma in situ | 3 |
| Illegible records | 32 |
| Missing records | 30 |
Aboriginal status by person and tumour characteristics.
| Characteristic | Non- Aboriginal | Aboriginal | p-value(Fisher’s Exact Test) | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Person characteristics | |||||
| Gender | |||||
| Male | 280 | 68.6 | 8 | 50.0 | 0.1692 |
| Female | 128 | 31.4 | 8 | 50.0 | |
| Age at diagnosis | |||||
| <40 | 12 | 3.0 | 2 | 12.5 | 0.1158 |
| 40-49 | 67 | 16.5 | 4 | 25.0 | |
| 50-59 | 100 | 24.6 | 6 | 37.5 | |
| 60-69 | 123 | 30.2 | 3 | 18.8 | |
| 70-79 | 66 | 16.2 | 0 | 0.0 | |
| 80-89 | 31 | 7.6 | 1 | 6.3 | |
| 90+ | 8 | 2.0 | 0 | 0.0 | |
| Metro/rural | |||||
| Metro | 307 | 75.3 | 5 | 31.3 | 0.0004 |
| Rural | 101 | 24.8 | 11 | 68.8 | |
| Country of birth | |||||
| Australia | 211 | 51.7 | 15 | 93.8 | 0.0006 |
| Other | 197 | 48.3 | 1 | 6.2 | |
| Smoker | |||||
| Yes | 154 | 37.8 | 7 | 43.8 | 0.8911 |
| No | 16 | 3.9 | 0 | 0.0 | |
| Missing | 238 | 58.3 | 9 | 56.3 | |
| Disease characteristics | |||||
| Stage | |||||
| I | 27 | 6.6 | 0 | 0.0 | 0.5867 |
| II | 52 | 12.8 | 1 | 6.3 | |
| III | 35 | 8.6 | 3 | 18.8 | |
| IV | 59 | 14.6 | 2 | 12.5 | |
| Missing | 235 | 57.6 | 10 | 62.5 | |
Aboriginal status and survival.
| Non-Aboriginal | Aboriginal | p-value(Log-rank test) | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Death from any cause (5 year follow-up period) | |||||
| Yes | 229 | 56.1 | 8 | 50.0 | 0.8685 |
| No | 179 | 43.9 | 8 | 50.0 | |
| Cancer related death or recurrence (5 year follow-up period) | |||||
| Yes | 209 | 51.2 | 7 | 43.8 | 0.8282 |
| No | 199 | 48.78 | 9 | 56.3 | |
| Cancer related death (5 year follow-up period) | |||||
| Yes | 179 | 43.9 | 6 | 37.5 | 0.7146 |
| No | 229 | 56.1 | 10 | 62.5 | |
Both gender and smoking showed no association with Aboriginal status. As expected, Aboriginal individuals were more likely to reside in non-urban areas (Table 2). Although Aboriginal individuals appeared to be more likely to present with oral SCC at a younger age and with a more advanced disease stage, neither of these trends were statistically significant (Table 2), most probably because of the small numbers of Aboriginal subjects (insufficient power to detect a difference).
There appeared to be no statistical difference in 5-year survival between Aboriginal and non-Aboriginal subjects. This was the case for: death from any cause; cancer related death or recurrence (indicator of treatment failure); and cancer related death (Table 3 and Figs. 1-3).
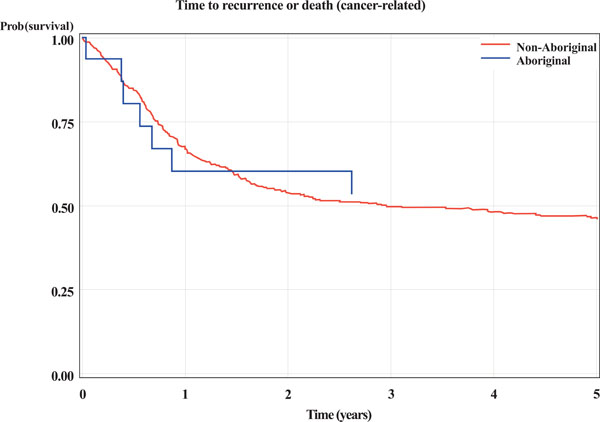
Kaplan-Meier curves for Aboriginal and non-Aboriginal status. The outcome is time (years) from initial biopsy to cancer related death or recurrence.
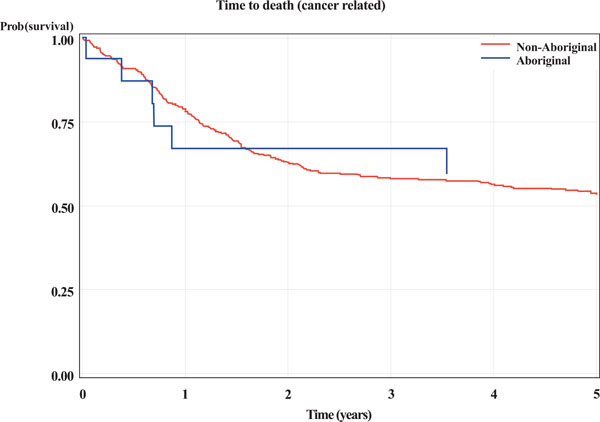
Kaplan-Meier curves for Aboriginal and non-Aboriginal status. The outcome is time (years) from initial biopsy to cancer related death.
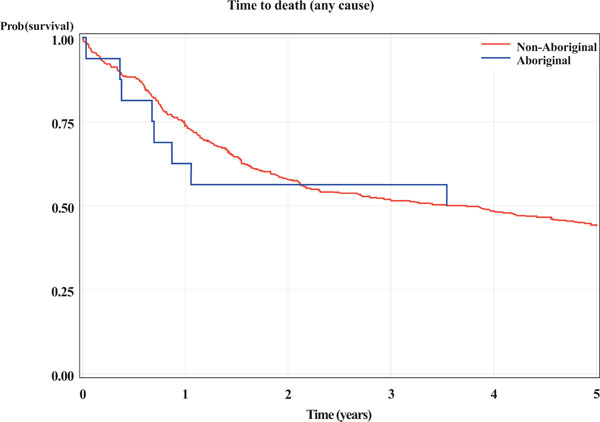
Kaplan-Meier curves for Aboriginal and non-Aboriginal status. The outcome is time (years) from initial biopsy to death from any cause.
DISCUSSION
Oral SCC is the most common type of malignancy affecting the oral cavity [1] with tobacco smoking and alcohol use constituting the two main risk factors for this disease. Tobacco smoke contains in excess of sixty carcinogenic chemicals with N-nitrosonornicotine, 4-(methyl-nitrosamino)-1-(3-pyridyl)-1butanone and polycyclic aromatic hydrocarbons, in particular, being linked to oral cancer [1]. Alcoholic drinks are predominantly composed of ethanol, glucose and water. Acetaldehyde, a product of ethanol metabolism, is a DNA mutagen and responsible for the oral carcinogenic effect of ethanol [1, 16]. Additional local and systemic oncogenic mechanisms attributed to ethanol intake include its ability to: act as a solvent and facilitate tissue entry of other carcinogens; damage cell membranes, increasing permeability; impair DNA repair mechanisms; impair carcinogen detoxification through hepatotoxicity; induce immunosuppression and predispose to malnutrition [16]. Some alcoholic beverages may also contain impurities such as N-nitrosodiethylamine and polycyclic aromatic hydrocarbons, further increasing their carcinogenic potential [16]. While Aboriginal individuals are at increased risk of substance misuse [12], surprisingly little is known about oral cancer in this population.
Over a ten year period from January 1990 to 31st of December 1999, we were able to identify only 16 Aboriginal individuals with oral SCC through the WACR. This small number can partially be explained by the very small Aboriginal population in Western Australia (3.8%) [10]. Concerns have also been raised in the literature regarding incorrect identification of Aboriginal individuals in cancer registries [10]. It is therefore possible that our figure of 16 individuals is an underestimation. Furthermore, oral cancer is a disease which predominantly affects older males, with the mean age of presentation occurring between the 5th and 8th decades of life [1].The significantly reduced life expectancy of Aboriginal individuals may therefore also contribute to low rates of oral cancer in this population [10].
We limited our study to individuals with no prior, concurrent or subsequent cancer. We have done this as another diagnosis of a malignancy may have adversely influenced survival of individuals diagnosed with oral SCC. However further studies are required incorporating these individuals with co-morbidity.
No association was observed between Aboriginal and non-Aboriginal status and sex, nor between Aboriginal and non-Aboriginal status and smoking. This may again be explained by the very small numbers of Aboriginal individuals identified in our study population.
While not statistically significant, the trend towards decreasing oral SCC diagnosis amongst Aboriginal population with advancing age is probably due to their shortened life expectancy, which is about 17 years lower than that of other Australians [10]. The consequent trend towards an increased chance of oral SCC at a younger age for Aboriginal subjects, may also be exacerbated by their significantly higher smoking rates and higher risk alcohol exposures [17]. This is in keeping with previously published data on smoking related cancers where excess incidence and mortality is more apparent in indigenous Australians under 65 years of age, than in other similar aged Australians [18].
As expected, most Aboriginal individuals diagnosed with oral SCC resided in non-urban areas. The remote residence of this population adversely impacts on their access to appropriate medical services. Problems with transport, accommodation arrangements and alienating city hospital environments are well recognised barriers encountered by rural and remote Aboriginal individuals [13]. While this may in part explain the possible trend towards Aboriginal individuals presenting with a more advanced disease stage, the five year survival was not significantly different between the Aboriginal and non-Aboriginal populations. The survival and recurrence outcomes for Aboriginal people should be monitored in future studies in case differences between Aboriginal and non-Aboriginal populations emerge.
This study is limited by the small numbers of Aboriginal individuals identified and by the quality of administrative records. Exclusion of a significant number of records from the final analysis was the result of incomplete hospital data and the importance of accurate medical records cannot be overemphasized. Data pertaining to the disease stage was particularly poorly recorded. For practical reasons extraction of medical records for data collection was limited to Western Australia’s three main public hospitals. Records of 11% of relevant cases identified through WACR were held in other institutions.
CONCLUSION
This study provided new information on person and disease characteristics of Aboriginal and non-Aboriginal Western Australian individuals diagnosed with oral SCC. Although the number of Aboriginal individuals was small, there appeared no difference between Aboriginal and non-Aboriginal subjects in the profile of the disease or 5-year survival. While this observational study is underpowered to detect moderate differences, this largely descriptive study provides data which have not previously been published. Access to the WACR data was pivotal in this study. The importance of accurate medical records is emphasized. Future studies involving Aboriginal Australians in particular could include larger scale, multicentre studies.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
The authors would like to thank the WACR staff for their assistance in this project.


