All published articles of this journal are available on ScienceDirect.
Immune Thrombocytopenic Purpura Presenting as Unprovoked Gingival Hemorrhage: a Case Report
Abstract
Immune thrombocytopenic purpura is an autoimmune disease characterized by auto-antibody induced platelet destruction and reduced platelet production, leading to low blood platelet count. In this case report, the clinical diagnose of a patient with immune thrombocytopenic purpura and spontaneous gingival hemorrhage by a dentist is presented. The patient did not have any systemic disease that would cause any spontaneous hemorrhage. The patient was referred to a hematologist urgently and her thrombocyte number was found to be 2000/μL. Other test results were in normal range and immune thrombocytopenic purpura diagnose was verified. Then hematological treatment was performed and patient’s health improved without further problems. Hematologic diseases like immune thrombocytopenic purpura, in some cases may appear firstly in the oral cavity and dentists must be conscious of unexplained gingival hemorrhage. In addition, the dental treatment of immune thrombocytopenic purpura patients must be planned with a hematologist.
INTRODUCTION
The oral cavity might be thought of as the mirror to the body since oral symptoms accompany many systemic diseases. Most of these oral symptoms are non-typical, but must be appropriately acknowledged for the probabilities of a concurrent hematological disease [1]. The oral symptoms of these diseases may be the first clinical introduction that warns the dentist to an underlying hematological disorder [2].
Immune Thrombocytopenic Purpura (ITP) is an acronym for primary immune thrombocytopenia, previously referred to idiopathic thrombocytopenic purpura. In medicine, purpura is a general term for reddish-purple skin lesions caused by bleeding in the dermis or subcutaneous tissues [3]. ITP is an auto-immune disorder characterized by auto-antibody induced platelet destruction and reduced platelet production, leading to a low peripheral blood platelet count [4]. Harrington et al. suggested that some factors in the plasma of ITP patients were destroying the platelets [5]. The same group later provided evidence of the plasma factor resided in the gamma-globulin fraction which suggests that it was in fact an antibody against platelets [6]. However, concepts surrounding the mechanisms of thrombocytopenia in ITP have shifted from the traditional view of increased platelet destruction mediated by autoantibodies to more complexmechanisms in which both impaired platelet production and T cell–mediated effects play a role [7].
In the absence of reliable predictive clinical or laboratory parameters of disease duration, the term “newly diagnosed ITP” was suggested for all cases at diagnosis whereas the term of ‘chronic ITP’ is used for the patients with ITP lasting for more than 12 months [8]. Recent epidemiologic data suggest that the incidence in adults is approximately equal for the genders except in the mid-adult years (30-60 years), when the disease is more prevalent in women [7]. In this case report, the early diagnose and treatment period of a patient with immune thrombocytopenic purpura and a complaint of spontaneous gingival hemorrhage, who was referred to Gulhane Military Medical Academy, Department of Periodontology, are presented.
MATERIALS AND METHODS: CASE DESCRIPTION
The 48-year-old female patient’s main complaint was gingival hemorrhage that lasted for 8 hours. The medical history revealed that the patient did not have any systemic disease and had never presented hemorrhage related disorders. Furthermore, the patient did not use any medication and no trauma history was present. Intra-oral examination revealed that she used full-mouth fixed bridge in the upper jaw and removable prosthesis and fixed bridge (anterior region) combination in the lower jaw. The patient reported that she did not brush her teeth regularly but it was observed that she did not have any periodontal disease that would cause any spontaneous hemorrhage (Fig. 1). The extra-oral physical examination revealed petechiaes and discoloration of the
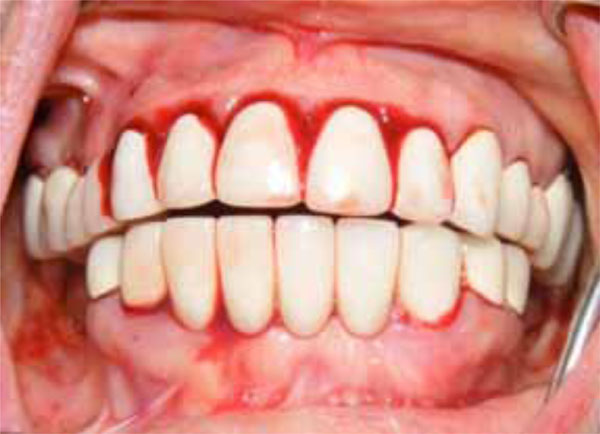
Initial oral aspect; Spontaneous hemorrhage.
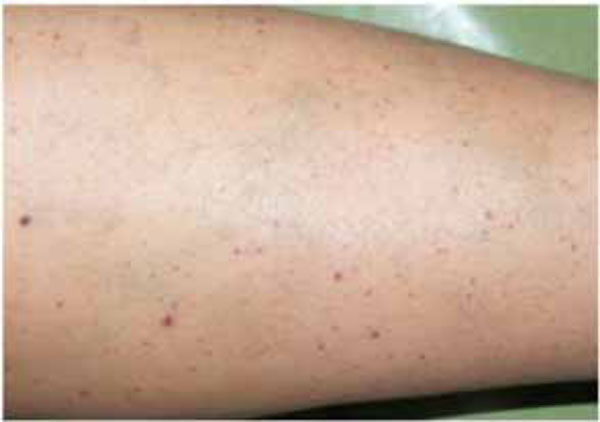
Petechiaes and discoloration of the skin, at the leg region.
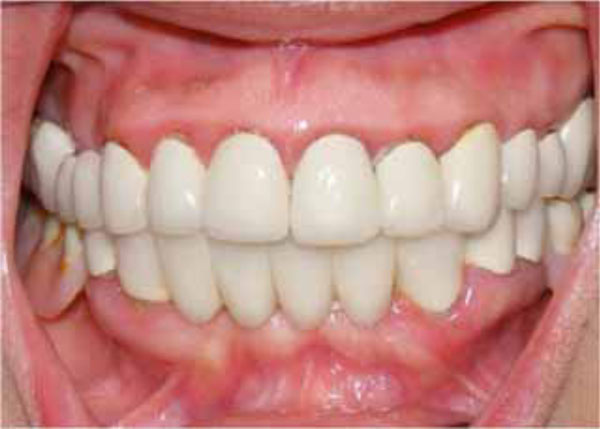
Final oral aspect.
skin were seen in the whole body, especially at the leg region. But the patient was not aware of these discolorations (Fig. 2). The patient was referred to a hematologist due to the prime suspect was thrombocytopenia. After the hematological tests, the patient’s platelet number was found as 2000/μL (Table I). The other hematologic test results (WBC, RBC etc) were in normal range. Immunologic tests (HBsAG, Anti-HBs, Anti-HCV, Anti-HIV, Anti-Cardiolipin IgM and IgG, Anti- Fosfotidil Serin IgG and IgM) results were negative. Also, International Normalized Ratio (INR), Fibrinogen, Partial Thromboplastin Time (PTT) values were in normal range. Hepatomegaly, splenomegaly and lympadenopathy were absent and ITP diagnose was verified. The patient received platelet replacement (infusion of 6 units of platelet concentrate), oral cortisone tablet (Methylprednisolone - 16 mg, 2 x 1, Prednol®, MN Pharmaceuticals, İstanbul, TR) and general care. The next day; following the administration of cortisone, the values did not differ compared to baseline: PLT: 3.000/μL, Hct: % 38,34 and MPV: 5,91 fL. After 8 days, new hematological exams revealed the following results: PLT: 223.000/μL, Hct: %39,76 and MPV: 6,99 fL (Table I). After hematological treatment, the patient’s health improved without further problems. The petechias diminished and ultimately disappeared, and the recovery of spontaneous hemorrhage was observed. Supra-gingival scaling and root planning were performed and oral hygiene instructions were given two weeks after the hematological recovery. The patient was motivated to practice regular tooth brushing with a soft-bristle toothbrush and 0.2% chlorhexidine digluconate oral rinse was prescribed. Also, the importance of using interdental brushes for her oral health was emphasized. The patient was placed under regular medical follow-up care and periodontal conditions were stable over 12 month follow-up period associated with the remaining teeth (Fig. 3).
Blood parameters.
| Tests | 1. Day | 3. Day | 8. Day | Reference Unit |
|---|---|---|---|---|
| Platelet (PLT) | 2 x 103/ul * | 3 x 103/ul * | 223 x 103/ul | 130- 400 x 103/ul |
| Mean Platelet Volume (MPV) | 4,00 fL * | 5,91 fL * | 6,99 fL | 6-13 fL |
| Red Blood Cell (RBC) | 4,21 x 106/ul | 4,31 x 106/ul | 4,39 x 106/ul | 3.8 – 5.1 x 106/ul |
| Hemoglobulin (Hgb) | 12.7 g/dl * | 12.9 g/dl * | 13,2 g/dl | 13-17 g/dl |
| Hematocrite (Hct) | % 38,2 | % 38,34 | % 39,76 | % 36-46 |
| White Blood Cell (WBC) | 6.5 x 103/ul * | 8.7 x 103/ul * | 13.1 x 103/ul * | 4.5 - 11 x 103/ul |
DISCUSSION
The diagnosis and management of spontaneous gingival hemorrhage can be confusing and may require a systematic medical approach [9]. Comprehensive history taking can give traces to exclude or include causes of gingival hemorrhage, such as periodontal disease, trauma, anticoagulant therapy, hematological disorders, or bacterial infection such as histoplasmosis or other stomatitis [10]. To the author’s knowledge, in spite of frequent involvement of the oral mucosa, only a few adult cases with ITP [11-14] and the pediatric cases with ITP that were referred from Hematology Departments to the dentists were reported in dental literature [15, 16]. Also, a case with steroid-induced damage to the condyles was caused by treatment for ITP [17]. A 77 year-old female patient with continuous intermittent post-extraction hemorrhage diagnosed with ITP was presented [18]. However, the present case with spontaneous gingival hemorrhage is the first reported case of the clinical diagnose of a patient with newly diagnosed ITP by a dentist and this patient was referred to the hematologist.
Oral manifestations of ITP include petechiae, ecchymoses or haematomas in easily traumatized areas such as buccal mucosa, lateral borders of the tongue, and the limit between the soft and hard palate [16]. Other findings are spontaneous gingival and mucocutaneous hemorrhage [9, 15, 16]. In the present case, oral lesions were not seen in the mouth but spontaneous gingival hemorrhage was observed.
The diagnostic hallmark of ITP is a low platelet count without identification of alternative causes of thrombocytopenia by history, besides physical and laboratory evaluations [19]. Platelet count has been the major parameter for predicting severity of ITP and risk of hemorrhage. Some with severe ITP (platelets less than 10,000/μL) do not bleed while others with higher platelet counts bleed excessively [20]. The destruction of platelets by antibodies causes a rise in the generation of young platelets that are more efficient in managing hemostasis [21] Therefore, hemorrhage symptoms are fewer in the patients with ITP and confronted in the patients with thrombocytopenia of other etiologies [22].Generally, ITP patients are asymptomatic in the presence of platelet counts that are greater than 50.000/μl [23]. In the present case, hematologic test results were in normal range, except the fact that platelet number (2000/μL) and spontaneous gingival hemorrhage were seen. However, gingival hemorrhage was not noticeable, so hematocrite and hemoglobulin values remained in normal range all the time. Hunter et al. reported that white cell count was normal and anaemia was present when significant blood loss occurred [24]. Also, corticosteroids could modulate both circulating blood lymphocyte subsets and the immune response [25]. Classic doses of corticosteroids were accepted to augment the number of leukocyte count and decreased the number of lymphocyte, eosinophile and monocyte [26]. In the present case, these parameters’ values were consistent with the literature.
Important possible diagnoses associated with thrombocytopenia, drug-induced thrombocytopenia, nutritional deficiency anemia, bacterial and viral infections (hepatitis and human immunodeficiency virus infection), von Willebrand disease, leukemia, aplastic anemia, hemolytic uremic syndrome, gestational thrombocytopenia, and congenital thrombocytopenias [19, 27]. It should be noted that many hematological diseases have oral manifestations like ITP: aplastic anemia, Glanzman thrombasthenia, Hemophilias, Ehlers Danlos Syndrome, Hyperglobulinemia and von Willebrand disease [19, 27].
Adequate dental plaque control techniques are the requisite in order to prevent inflammation, gingival hemorrhage and infection in ITP patients [15]. The patients with coagulopathies may ignore their oral hygiene due to the fear of hemorrhage during tooth brushing and flossing, which leads to increased periodontal problems and caries [28]. In our case, fixed bridges were used in the upper and lower jaws so the patient was encouraged to practice regular dental brushing with a soft-bristle tooth brush and to use a soft interdental brush and superfloss. A 0.2% chlorhexidine digluconate oral rinse was prescribed as well. Periodontal examination and scaling can be done normally without the risk of significant hemorrhage in the patients with coagulopathies. Also, ultrasonic instrumentation may result in less tissue trauma [28]. In our case, supragingival scaling was done after the hematological treatment because of its emergency.
Dental surgical procedures or even nerve-block anesthetic injections in the patients with platelet counts under 30.000/μL required consultation with a hematologist[29]. American Society of Hematology recommended the first-line treatment for ITP patients; use of corticosteroids (dexamethasone, methylprednisolone, prednisone) or intravenous infusion of immunoglobulin or intravenous infusion of anti-D when the platelet count is under 30,000/μL [7]. Dental treatments should perform with a platelet count over 50.000/mm3 and in consultation with the patient’s hematologist. Primary mucosal closure of surgical wounds and products (surgical stents, absorbable gelatine sponges, electrocautery and bone wax) should be utilized to achieve an adequate haemostasis for the minor oral surgical operations [16]. The drugs with anti-platelet aggregation effect which may interfere with haemostasis (acetylsalicylic acid and nonsteroidal anti-inflammatory drugs) must be avoided [28]. Also, antibiotics usage with treatment minimizes the risk of postoperative infections [16].
CONCLUSION
The symptoms of hematologic diseases like ITP may initially emerge in the oral cavity and they might be diagnosed by a dentist. Therefore, dentists must carefully examine the mouth and must be conscious of unrealized gingival alterations and hemorrhage. Consultation with the hematologist is essential for planning dental treatment of ITP patients and determining the severity of the disorder. Also, adequate oral hygiene methods and regular controls are essential for these patients in order to prevent inflammation, hemorrhage or the need for any aggressive treatment in the next phase.
CONFLICT OF INTEREST
The author confirms that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


