All published articles of this journal are available on ScienceDirect.
Peripheral Desmoplastic Ameloblastoma in Adolescent Age: Clinico-Pathological and Immunohistochemical Analisys of a Case
Abstract
The Extraosseous or Peripheral Ameloblastoma (PA) is a rare and benign odontogenic tumour, representing 1% to 5% of all ameloblastomas. It is usually localized in the soft oral tissues, without deep bone involvement. Its biological behaviour is specific, and several authors define PA as a non-infiltrating hamartomatous lesion. Indeed, recurrences rarely occur and progression in malignant tumors appears to be rare.
The PA originates from the tooth-forming apparatus and it consists of proliferating odontogenic epithelium, exhibiting the same histological cell types and patterns of the intraosseous counterpart or infiltrating ameloblastoma.
The peripheral desmoplastic ameloblastoma (PDA) can be classified as a newly recognized and very rare histological variant. To our knowledge, only a few cases of adult patients affected by PDA have been published.
The aim of this paper is to report a case of PDA affecting an adolescent patient. The clinical-pathological and immunohistological features are discussed in order to improve knowledge regarding a correct diagnosis and to differentiate PDA lesions from similar diseases.
INTRODUCTION
A 30-years period analysis of oral and maxillofacial adult patient pathologies revealed a 0.8 % prevalence of odontogenic tumors among all oral and maxillofacial pathologies, showing numbers in the range of 331 lesions out of 44,077 diagnostic categories analysed between the period 1973-2002 [1].
The Ameloblastoma and the odontoma are the most commonly recorded benign odontogenic tumors [2]. Ameloblastoma accounts for approximately 10% of odontogenic neoplasms and 1% of all oral and maxillofacial pathologies [3]. The neoplasm can occur in all ages and literature reports an higher frequency among patients aged from 30 to 50 years old [4, 5].
The current update regarding the ameloblastoma epidemiological diffusion underlines how 10%-15% of the recorded cases affects children or adolescents [6, 7].
Ameloblastoma usually occurs in the jawbones, but tumors characterized by similar histological features have been reported in gingival tissues. This group of diseases is referred to as peripheral, extraosseous or soft tissue ameloblastoma [8]. The peripheral ameloblastoma (PA) is a rare variant, representing approximately 2% to 10% of all ameloblastomas. In particular, it occurs in a significantly higher age group than does intraosseous ameloblastoma, being a rarity in adolescence [1, 5, 8]. The PA is a painless, firm neoplasm with exophytic growth. Size ranges from 0.3 to 4.5cm in diameter with a mean of 1.3cm. The surface is usually smooth but in several cases it has been described as “granular” or with a papillary or warty appearance. The colour of the lesion ranges from “pink” to “dark-red”. Its histological patterns are similar to intraosseous neoplasm: mainly follicular and acanthomatous, less common plexiform and basalioid configurations are observed [9, 10].
No radiological evidence of bone involvement is usually detected. The PA evolves through superficial bone erosion, known as “cupping” [11]. A more complex tumor is classified as Desmoplastic ameloblastoma (DA). It is a newly recognized histological variant of ameloblastoma, first described by Eversole in 1984 [12]. Histologically, a hypocellular desmoplastic stroma consisting of dense collagen fibers compressing the neoplastic cells into narrow islands is typical of these lesions. Although it was originally considered a central neoplasm involving the maxilla more than the mandible, few cases of peripheral desmoplastic ameloblastoma (PDA) exclusively affecting adult or elderly patients have recently been published [13-15].
Here, the a case of PDA affecting the (vestibulo-alveolar) mucosa of the maxilla in a 17-years old patient is described, while discussing its clinico-pathological and immunoistochemical features, and reviewing the literature on this topic.
CASE REPORT
A 17 years old male patient was referred by his dentist to the Oral Surgery Unit of our dentistry department with a clinical diagnosis of epulis. The patient was a no smoker and his anamnesis revealed generic health condition.
The patient noticed the presence of a painless red swelling on the vestibular gingiva of the left maxillary bicuspid region by four months. At that time, the lesion had showed a slow nodular growth. (Fig. 1).
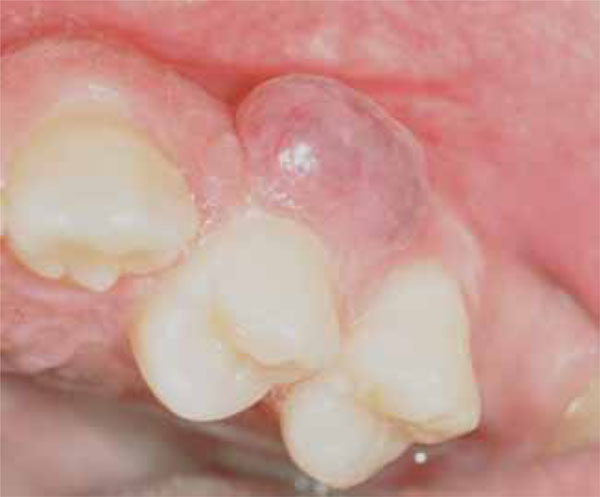
Intraoral photograph of soft tissue mass located on the vestibular gingiva in the left maxillary premolars area.
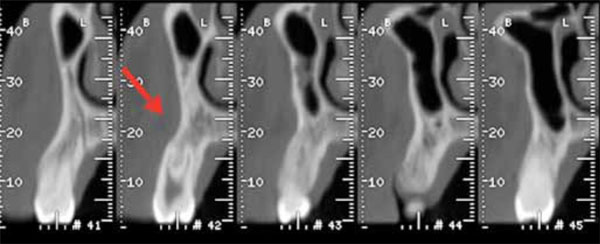
TC cross-section scans showing a mild depression in the vestibular plate (cupping) of the left premolar maxillary alveolar process.
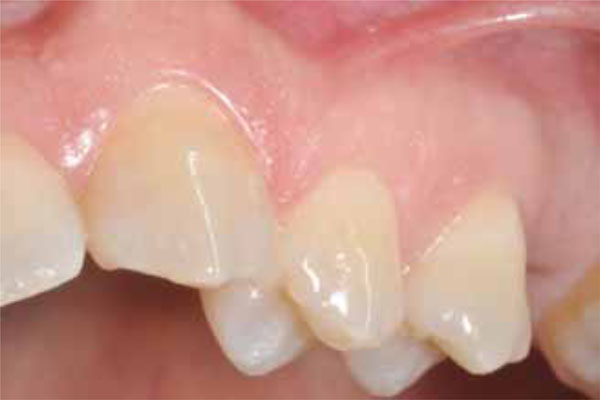
Intraoral photograph, taken thirty months after surgery: here, the healed vestibular gingiva without any tumor recurrence is shown.
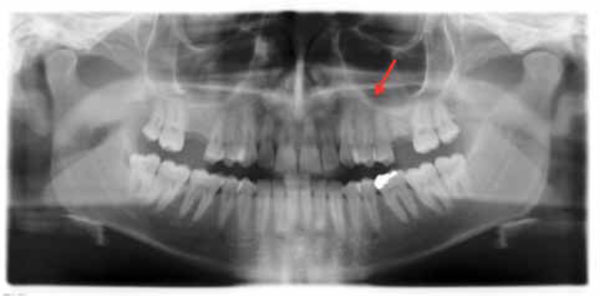
Ortopantomograph, thirty months after surgery, showing the intact bone in the left premolar maxillary area.
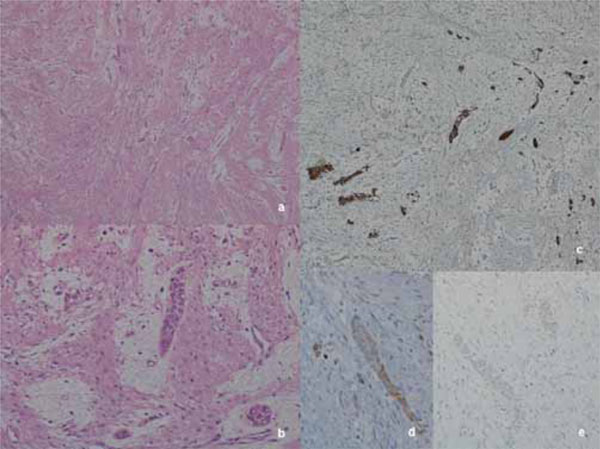
Histological low magnification shows dense spindle cells proliferation arranged in intersecting fascicles with scattered, isolated, thin, epithelial islands (a, Hematoxilyn-Eosin, 20x). High magnification emphasizes the epithelial neoplastic cells enveloped in a ring of mixoid substance inbetween the desmoplastic fascicles (b, Hematoxilyn-Eosin, 100x). Immunohistochemical staining for CKAE1/AE3 highlights the epithelial neoplastic strands compressed by the abundant collagen fibers (c, Hematoxilyn counterstain, 40x), while Bcl- 2 and CK 19 (d - e, Hematoxilyn counterstain, 100x): positive results in the epithelial neoplastic cells are rare, and very weak.
The clinical examination revealed a 2 cm red gingival neoplasm, localized on the vestibular gingiva of the upper left premolars, covered by normal epithelium with a smooth surface and being of a firm consistency. Oral hygiene status was good and there was no bleeding on probing. There were also no neurological function alterations and no obvious lymphadenopathy. All the involved teeth were vital. The 1 months-before TC-scan did not show any significant radiographic involvement of the alveolar bone except a mild depression in the vestibular plate (cupping). (Fig. 2).
Due to a clinical diagnosis of fibrous epulis, the patient was admitted to the hospital for an excisional biopsy including the underlying periosteum, with a narrow margin. A periodontal pack was placed on the wound to protect the exposed bone.
The surgical specimen, fixed in 10% formalin, was submitted for a histopathological examination in our Pathology Unit.
The lesion healed uneventfully, without clinical and radiological signs of recurrence at 30 months follow up. (Figs. 3, 4).
HISTOLOGICAL AND IMMUNOHISTOCHEMICAL EXAMINATION
The specimen magnification scansion shows a dense, spindle cell proliferation, with ulcerating superficial mucosa. The sample results embedded and compressed by the stromal component and a higher magnification emphisized scattered small nests and thin strands (occasionally islands) of epithelial neoplastic cells. The mono or bi-layered epithelial cells are mainly cuboidal in shape, with scanty cytoplasm and monomorphic bland nuclei (Fig. 5). In the rare-occuring islands, the peripheral cells could hint at a columnar phenotype with nuclear polarity; these islands have a very small, swirled, hypercellular centre with spindle-shape epithelial cells.
The stromal component is structured with a dense proliferation of fibroblasts, producing abundant collagen fibres arranged in large short crossing fascicles. A narrow zone of loose-structured connective tissue occasionally surrounds the epithelial nests and strands (Fig. 5).
Immunohistochemical staining for CKAE1/AE3 highlights the neoplastic epithelial component enveloped in the collagen fibres. The epithelial cells also show a weak, focal positivity for CK19, a weak but diffuse positivity for Bcl-2, while Ber-EP4 and EMA are negative (Fig. 5, CKAE1/AE3, CK19, Bcl-2).
The diagnosis of peripheral desmoplastic ameloblastoma was made.
DISCUSSION
When odontogenesis was completed, residual epithelial cells remained scattered in the soft tissue adjacent to the tooth. In some cases, these quiescent embryonic residues of odontogenic epithelium may return to an active state, capable of hamartomatous or related to a neoplastic proliferations [16].
These events could explain the pathogenesis of PA and several probable sources of this lesion are recognized: remnants of the dental lamina, the so-called “glands of Serres,” odontogenic remnants of the vestibular lamina, pluripotent cells in the basal cell layer of the mucosal epithelium, and pluripotent cells from minor salivary glands [17].
Peripheral ameloblastoma exhibits the same histomorphologic cell type and patterns seen in intraosseous or infiltrating ameloblastoma: they share the microscopical features defined by the World Health Organization: (1) islands with peripheral columnar cells, surrounding central ones and resembling the stellate reticulum; (2) the peripheral cells are hyperchromatic, lined up in a palisaded fashion, their nuclei are displaced away from the basement membrane and the cytoplasm are vacuolated; (3) the central cells are loosely arranged and often become cystic [18].
The recently recognized Desmoplastic Ameloblastoma is considered a rare variant of central ameloblastoma. It has been included in the World Health Organization’s Histopathological Typing of odontogenic tumors, similar to benign, locally invasive tumor with low recurrence rate and peculiar histological features, characterized by epithelial changes due to the pressure of stroma on epithelial tissue [19, 20].
Histogenesis of Desmoplastic Ameloblastoma is not clarified. Kishino and Kawai assumed the origin to be from the periodontal ligament, thanks to the oxitalan fibres detected in the stroma of this neoplasm [21, 22]. Philipsen et al. suggested the origin to be from the remnants of dental lamina located in gingival tissues [23].
However, these authors outlined biological behaviour as not well known, due to the low number of cases reported (about 145) [24].
The Peripheral Desmoplastic Ameloblastoma is a rare event, reported in only four cases involving adult patients. This neoplasm shows the clinical features of a non invasive, slow growing mass of soft tissue covered by normal mucosa of the jaws.
To the best of our knowledge, the patient in this study represents the a rare case of PDA in adolescent age. Due to the clinical presentation (exophytic growth on the soft tissues overlying the tooth-bearing areas of the jaws, with uninvolved underlying bone) the inital diagnosis was Fibrous Epulis. The lesion was treated by local excision, extended to 2-3 mm to ensure negative margins. The supra-periosteal surgical approach resulted as decisive because of the histological diagnosis of desmoplastic peripheral ameloblastoma. In fact, contrary to intraosseous counterpart (which is locally aggressive with bone destruction and requiring extensive surgical handling) the PDA does not manifest such behaviour and therefore, needs a conservative treatment.
The differential diagnosis included fibrous epulis, odontogenic gingival epithelial hamartoma, basal cell carcinoma, and peripheral odontogenic fibroma. Fibrous epulis, the first clinical diagnosis, can be easily differentiated by histology due to the lack of an epithelial component and a constant presence of an inflammatory reaction. Regarding odontogenic gingival epithelial hamartoma, the question is whether this lesion should be included under the histopathological spectrum of the peripheral ameloblastoma because of the overlapping clinicopathological features, that is the present view [12, 25].
The basal cell carcinoma could be also escluded in this case because of clinical setting (the young age of the patient; absence of a syndromic set) and the histological and immunohistochemical features (Ber-EP4 negative epithelial neoplastic cells, useful in differential diagnosis) [26].
The peripheral odontogenic fibroma certainly constitutes the most important histopathologic differential diagnostic issue; the proliferation of strands and islands of odontogenic epithelium in this tumour may be so extensive as to make the distinction from PDA very difficult. Gardner and Ng investigated the immunohistochemical characteristics of both neoplasms in an attempt to elucidate their histogenesis but they could not confirm or exclude an origin in the surface epithelium for the epithelial elements [20, 27].
As suggested by Kumamoto et al. the frequent CK19 negativity of the neoplastic cells, usually at great length positive in the classical variants of ameloblastoma, might represent a sort of de-differentiation from odontogenic epithelial characteristics [28].
This case study also results negative for CK19, except for rare, focal, very weak positivity (Fig. 5, CK19).
However the differential diagnosis related to the PDA concerns benign neoplasms and/or hamartomatous lesions requiring only conservative treatment modalities, with the exception of basal cell carcinoma in older patients or in the set of Gorlin syndrome.
In conclusion, this paper not only reports the currently fifth case of PDA but also the first presented by a young patient. Further reports of new cases are needed to increase knowledge on the biological behaviour and the clinical treatment of this rare variant of odontogenic tumors.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Authors would like to express their gratitude to Prof Veronica Gavin, B.Sc; T.E.F.L. for her precious contribute on giving English language proof to this manuscript.


