A Keratocyst in the Buccal Mucosa with the Features of Keratocystic Odontogenic Tumor
Abstract
A 74-year-old male patient consulted us for an elastic firm mass in the right buccal mucosa. CT examination revealed a well-circumscribed oval cystic lesion in the anterior region of the masseter muscle. On MRI, the lesion showed a low signal on T1-weighted image and a high signal on T2-weighted image. Aspiration biopsy demonstrated the presence of squamous cells in whitish liquid. Under the diagnosis of epidermoid cyst, the lesion was intraorally extirpated under general anesthesia. The lesion was cystic at the size of 30 × 25mm. Histologically, the cyst wall was lined with parakeratinized squamous epithelium corrugated on its surface, the basal layer of which consisted of cuboidal cells showing palisading of the nuclei. Immunohistochemically, the lining epithelium was positive for CK17 and negative for CK10. The basal and suprabasal cells were labeled for Ki-67 at a relatively high rate. These features are compatible with those of keratocystic odontogenic tumor.
INTRODUCTION
Keratocystic odontogenic tumor (KCOT) is one of the most common odontogenic tumors of ectodermal origin. It is categorized as a benign odontogenic tumor in the WHO classification 2005 [1] because of its neoplastic potential and high recurrence rate. KCOT generally originates from the remnant of dental lamina or from basal cells of the oral epithelium [1]. It predominantly develops in the mandible or maxilla [2, 3], and occasionally on the gingiva as a peripheral type of manifestation [3, 4]. Interestingly, keratocysts in the buccal mucosa with similar histological features of KCOT have been reported in the recent literature [5-7]. It has not been proven whether these cysts are really odontogenic or originated from other tissues [7]; therefore, a case of keratocyst in the buccal mucosa is worthy of attention.
In this report, we present a case of keratocyst developing in the right buccal mucosa with the features of KCOT.
CASE REPORT
A 74-year-old man consulted our clinic with a complaint of swelling in the right buccal region. The patient noticed the swelling 5 years ago and had received aspiration therapy repeatedly to reduce the swelling. He was generally healthy and had no medical history. On examination, an elastic firm, movable mass of 50mm was palpable in the right buccal region (Fig. 1). The overlying mucous membrane was normal.
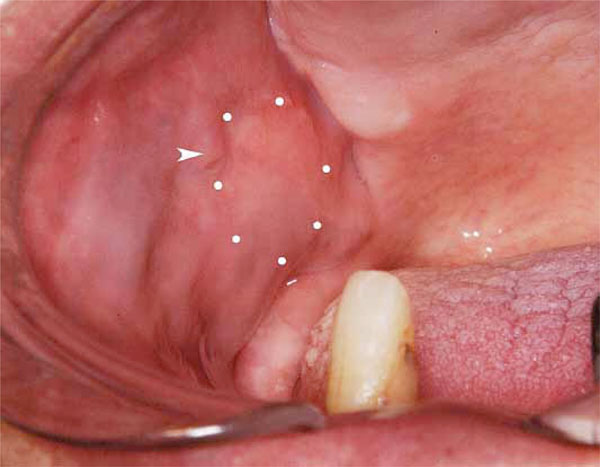
Intraoral finding
Swelling is observed at the right buccal mucosa (dotted area) posterior to the orifice of Stensen’s duct (arrow head).
The mass was not adhesive to either the mucous membrane or the skin. The maxilla was totally edentulous and only bilateral canines and the left first premolar were present in the mandible. Hypoesthesia or palsy of the right face, trismus, swelling of cervical lymph nodes or the right parotid gland was not observed. CT examination revealed a well-circumscribed oval cystic lesion of 35 mm with the density slightly less than that of muscle in the anterior region of the masseter muscle (Fig. 2a). On MRI, the lesion showed a low signal on the T1-weighted image and a heterogeneous high signal on the T2-weighted image including intermediate signal in the under portion (Fig. 2bc). Aspiration through the buccal skin revealed whitish liquid with squamous cells and shadow cells with a few neutrophils and lymphocytes in the cytological examination.
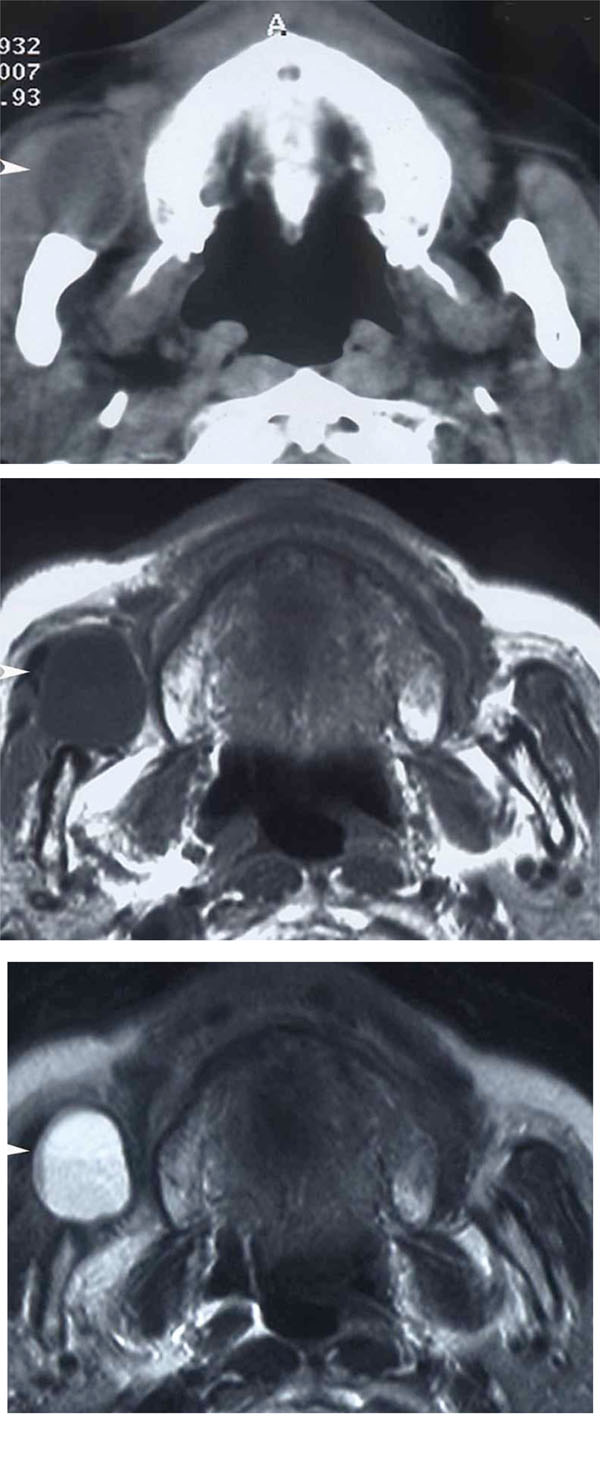
Imaging
a: Axial CT, b: Axial MRI (T1WI), c: Axial MRI (T2WI)
A well-circumscribed low density mass is observed in the right buccal region anterior to the masseter muscle in CT (arrow head). It shows a low signal on T1-weighted MRI and a heterogeneous high signal on T2-weighted MRI (arrow head).
The clinical diagnosis of epidermoid cyst at the right buccal mucosa was made. The lesion was intraorally extirpated under general anesthesia through the incision along the anterior border of the mandibular ramus (Fig. 3). Detachment of the lesion from the surrounding tissue was relatively easy. Stensen’s duct was not related to the lesion. The wound was closed after placing a silicon drain. Wound healing is good and no sign of recurrence has been observed for more than 4 years after the surgery.
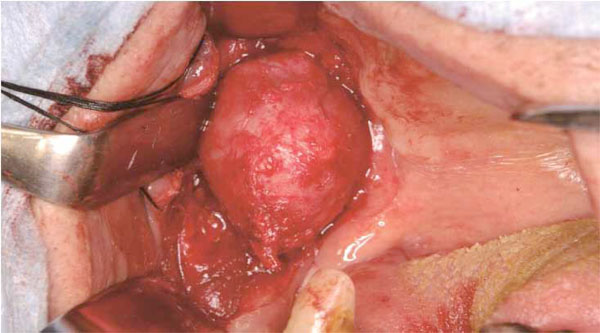
Operative finding
A well-circumscribed mass is exposed anteriorly to the masseter muscle.
The lesion was cystic, 30 × 25mm, weighed about 6g and contained brown serous liquid with a few cellular debris or keratin scale (Fig. 4). Histologically, the lesion was lined with squamous epithelium with parakeratinization corrugated on the surface (Fig. 5a). The basal layer consisted of cuboidal cells showing palisading of the nuclei and a smooth border (Fig. 5b). No daughter cysts, epithelial islands, hair
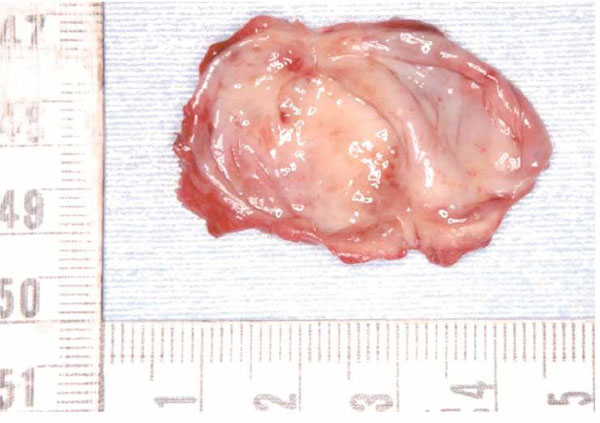
Extirpated lesion
The lesion is cystic and covered with a thin smooth wall.
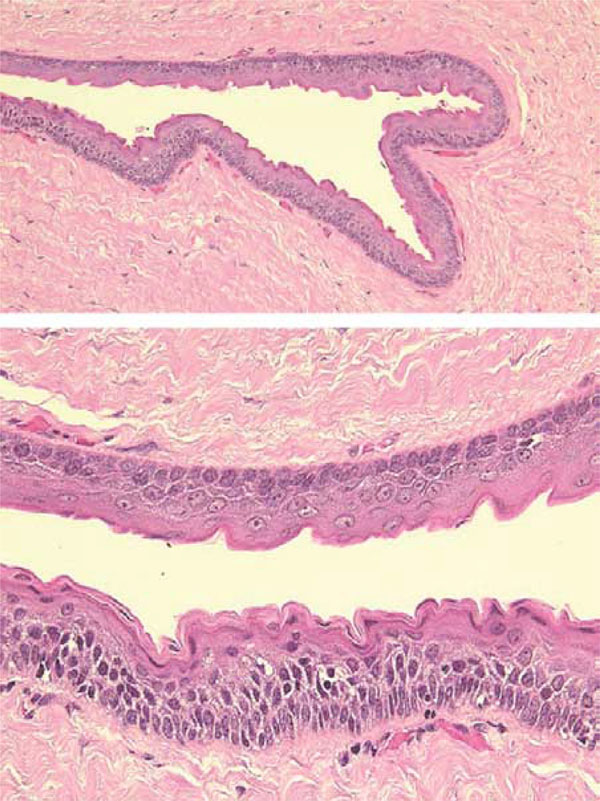
Histological findings
a: The cyst wall is lined with parakeratinized squamous cells with a corrugated surface.
b: Nuclei of the cells in the basal layer are palisaded. The rete ridge of the epithelium is not evident.
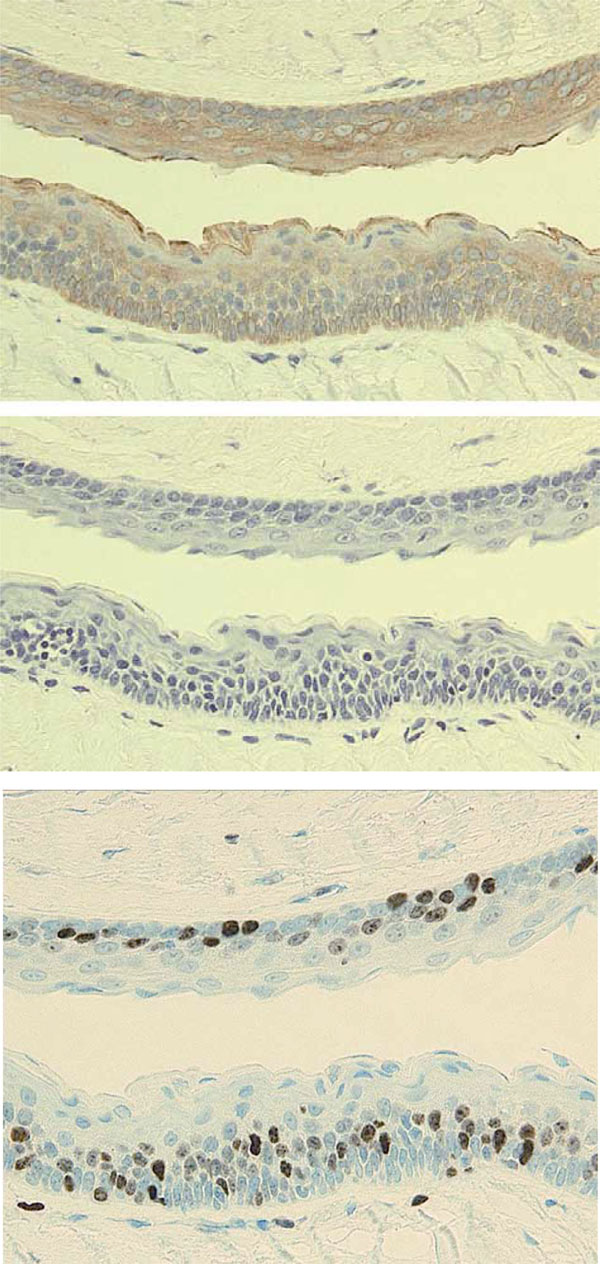
Immunohistochemical findings
Immunohistochemical staining was performed using serial sections of the specimen shown in Fig. (5b).
a: CK17 (E3; Dako)
b: CK10 (DE-K10; Dako)
c: Ki-67 (MIB-1; Dako)
DISCUSSION
The diagnosis of KCOT is based on its characteristic histological features [8] and is not difficult for a typical keratocyst in the jawbone; however, the diagnosis of KCOT is controversial for a keratocyst in the buccal mucosa, even though it has similar histological features to KCOT. Since keratocysts in the buccal mucosa can originate from either odontogenic or epidermal tissue, a definite diagnosis cannot be made in the absence of the obvious evidence of the originating tissue. Several lesions should be differentiated from KCOT.
Epidermoid cyst with similar histological features to KCOT may develop in the buccal mucosa; however, there are several differences between KCOT and an epidermoid cyst. The preferred site of KCOT is apparently unrelated to the line of closure of the embryonic fusion plane. Histologically, the lining epithelium of KCOT shows characteristic palisading of cuboidal basal cells and parakeratinization with a corrugated surface. A different pattern of cytokeratin expression is also helpful for differential diagnosis [7]. CK10, which is specific to a cornified surface such as skin, was negative in KCOT. On the other hand, CK17 was positive in KCOT, but negative in epidermoid and orthokeratinized odontogenic cysts [9]. Ki-67-labeled cells were higher in KCOT, suggesting higher proliferation potential. Predominant suprabasal distribution of Ki-67-labeled cells is also characteristic in KCOT.
Keratocyst originating from skin adnexa such as hair follicles and sebaceous glands may ectopically occur in the buccal mucosa [7]. In addition, cutaneous keratocyst, which is generally prevalent in patients with nevoid basal cell carcinoma syndrome [10, 11], can also develop independently of the syndrome [12]. KCOT can be differentiated from cutaneous cystic lesions such as trichilemmal cyst and steatocystoma by its catagen pattern and the presence of pilosebaceous units, respectively [12], even if these develop in the buccal mucosa; however, cutaneous keratocysts resembling steatocystoma and lacking pilosebaceous units are quite difficult to differentiate from KCOT, since the corrugated anagen-like lining of these keratocysts is quite similar to that of KCOT [13]. Furthermore, the lining epithelium of steatocystoma, not of sebaceous cells, shows a similar pattern of cytokeratin expression to KCOT [7,14]. Cutaneous keratocysts rarely occur because once a true follicular cyst develops from the isthmus of a hair follicle, its lining possibly adopts a catagen pattern [13]; however, the ectopic development of a cutaneous keratocyst in the buccal mucosa cannot be completely excluded.
The mechanism by which KCOT can develop in the buccal mucosa is not well known. Since KCOT is considered to originate from the remnants of dental lamina, these cells should be displaced in the buccal mucosa and persist during embryogenesis [8]. Recent embryological reports [15,16] describing the developmental relationship between deciduous dentition and the oral vestibule may give an important clue. According to these papers [15,16], the vestibular structure involved in the formation of the oral vestibule and buccal mucosa is reiteratively intermigrated with the dental epithelium around the upper molar areas during tooth formation. These findings suggest that the remnants of dental lamina entrapped in the buccal tissue during embryogenesis may develop a keratocyst with the features of KCOT. Other odontogenic lesions such as ameloblastoma and odnotoma developed in the buccal mucosa were also reported [17, 18]. This hypothesis can explain why these odontogenic lesions in the buccal mucosa uniformly develop around the parotid papilla.
In summary, a keratocyst in the buccal mucosa with the features of KCOT is reported. Although a definite diagnosis of KCOT cannot be made, an odontogenic origin is suspected from the histological features and the immunohistochemical pattern of the lesion.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
ACKNOWLEDGEMENTS
Declared none.


