Effect of Toothpastes with Different Abrasives on Eroded Human Enamel: An in situ/ex vivo Study
Abstract
The aim of the present study was to investigate the abrasive effect of CaCO3 and SiO2-based fluoride-free experimental toothpastes on eroded human permanent dental enamel and evaluate the effectiveness of waiting periods between acid exposure and tooth brushing. Twelve volunteers wore palatal appliances containing human enamel blocks for two periods of five days each. The appliances were immersed in a soft drink for five minutes four times a day (9:00 am, 11:00 am, 2:00 pm and 4:00 pm). On two occasions, two blocks were not submitted to additional treatment; two blocks were brushed (30 s) either with a CaCO3 or SiO2 toothpaste immediately after erosion and two blocks were brushed 1 h after erosion. Thus, the sample was divided into six groups: erosion alone (CaCO3 and SiO2 control); brushing with fluoride-free toothpaste (CaCO3 immediate and 1 h after erosion; SiO2 immediate and 1 h after erosion). Significant differences in wear depth were found between the enamel blocks in the CaCO3 immediate and 1 h after erosion groups and the blocks in the CaCO3 control group (p=0.001; p=0.022). No significant differences were found regarding the change in roughness and wear depth between blocks submitted to immediate abrasion and 1 h after erosion (CaCO3 and SiO2). The data revealed that surface roughness and wear depth is increased when erosion is combined with dental abrasion, regardless of the abrasive used. Waiting for 1 h to brush the eroded blocks offered no protective effect.
INTRODUCTION
Studies have demonstrated that eroded tooth enamel is more prone to abrasion than sound enamel [1-3]. Dental erosion leads to the irregular loss of tooth volume as well as the softening of the surface to a depth of several microns. This softened zone is highly susceptible to physical forces [4].
The interaction between erosion and abrasion leads to greater dental surface wear in a shorter time, which has consequences in terms of function, sensitivity and esthetics [5]. To minimize additional harmful effects on the eroded tooth surface, toothpastes with different degrees of abrasiveness [2,6], fluoride compounds [3], fluoride concentrations [6] and various time intervals between erosion and brushing have been investigated. In situ studies have revealed that wear is decreased only by 25 to 30% after a one-hour wait [1,7] and is still increased 7- to 10-fold in comparison to non-brushed eroded specimens [1]. Even waiting two hours before brushing with fluoride-free toothpaste has no protective effect [8].
Studies assessing eroded enamel abrasion have compared fluoride and non-fluoridated toothpastes with the same abrasive agent [6, 8, 9]. However, little is known regarding the effect of different abrasives in non-fluoride toothpastes. Therefore, the purpose of the present study was to investigate the abrasive effect of fluoride-free experimental toothpastes containing calcium carbonate (CaCO3) and silicon dioxide (SiO2) on human permanent tooth enamel eroded by a carbonated soft drink and the effectiveness of waiting periods between acid exposure and tooth brushing.
MATERIALS AND METHODOLOGY
Ethical Considerations
This study received approval from the Human Research Ethics Committee of the Universidade Federal de Santa Catarina (Brazil) (Protocol number 195/05).
Sample Calculation
The parameters used for the calculation of sample size were a 5% significance level, 95% statistical power, standard deviation of 0.7505 µm and minimum difference of 0.9 µm in the abrasion detected between groups [10]. A minimum of 18 blocks was determined for each group, totaling 108 blocks (for each phase), to which six blocks were added to each group (144 blocks in each phase) to compensate for possible losses.
Volunteers
Twelve healthy adult volunteers (10 women and 2 men; mean age: 28 years) were recruited among students of dentistry. The inclusion criteria were adequate physiological saliva flow rates (stimulated > 1 ml/min; non-stimulated > 0.25 ml/min) [11], good oral health status (no cavities, significant gingivitis/periodontitis or visible plaque) and an absence of orthodontic appliances. The exclusion criteria were general/systemic illness, medication likely to interfere with saliva secretion and pregnancy. All participants agreed to participate in the study by signing a statement of informed consent.
Experimental Design
The in situ/ex vivo study was a randomized, double-blind, in situ crossover design carried out in two five-day periods. The following aspects were evaluated: eroded enamel, eroded/abraded enamel with experimental toothpastes and periods between erosion and abrasion (immediately and 1 h after erosion). The experimental toothpastes had similar formulation, except for the abrasive (CaCO3 or SiO2). The CaCO3-based dentifrice was composed of CaCO3, sodium lauryl sulphate, glycerine, hydroxyethyl cellulose, sodium saccharin, menthol, nipagin and distilled water. The SiO2-based dentifrice was composed of SiO2, sodium lauryl sulphate, glycerine, carboxymethyl cellulose, saccharin, titanium dioxide, sodium methyl-p-OH-benzoate, sodium trimetaphosphate, menthol and demineralized water. According to data furnished by the manufacturers, the abrasives used (CaCO3, Henrifarma, São Paulo, Brazil; SiO2, Tixosil 73, Rhodia, São Paulo, Brazil) have a medium degree of abrasiveness. The shape and size of the particles are displayed in Fig. (1 and 2). The volunteers wore a palatal appliance containing six blocks of permanent human enamel. In the first phase of the experiment, the volunteers were randomly chosen to receive either the CaCO3 or SiO2-based toothpaste; the toothpastes were crossed over in the second phase. Each phase lasted five days. The following groups were studied: CaCO3 and SiO2 control groups (erosive challenge alone), CaCO3 and SiO2 immediate groups (brushing with either CaCO3 or SiO2 toothpaste immediately after erosion) and CaCO3 and SiO2 after 1 h groups (brushing with either CaCO3 or SiO2 toothpaste 1 h after erosion) (Fig. 3). The volunteers received written instructions and a schedule.

Particles of CaCO3 abrasive.

Particles of SiO2 abrasive.
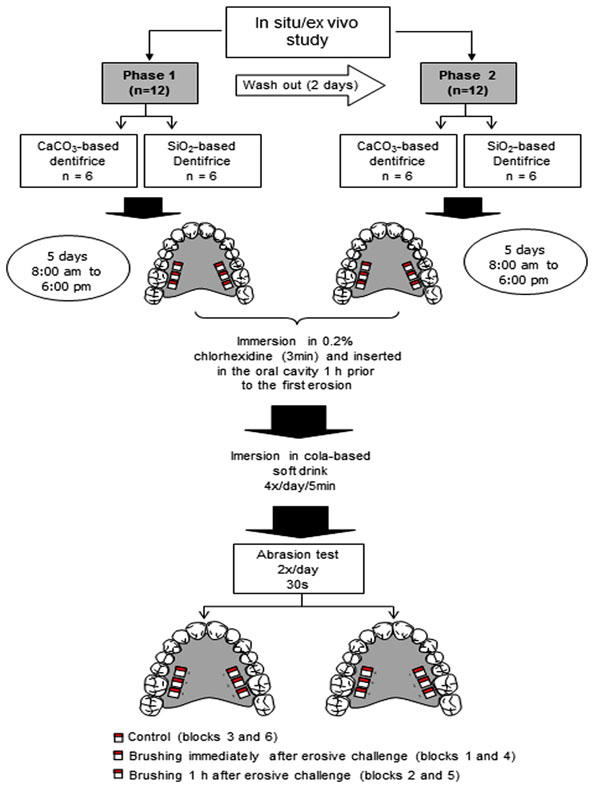
Schematic illustration of in situ/ex vivo study; Groups: CaCO3 control; SiO2 control; CaCO3 immediate; SiO2 immediate; CaCO3 after 1 h; SiO2 after 1 h.
The response variables were expressed in enamel roughness (Ra, µm), change in roughness (final Ra minus initial Ra) and depth of enamel wear.
Preparation of Enamel Blocks
Enamel blocks (4 x 4 x 2 mm) were obtained from the buccal and/or palatal surfaces of non-erupted human third molars, which were stored in a 0.1% thymol solution (pH 7.0) at room temperature until use.
Blocks for the in situ/ex vivo study were fixed to acrylic bases. The surface enamel of the blocks was ground flat with water-cooled 600-grit and 1200-grit silicon carbide paper (Norton, Vinhedo, Brazil) and polished with a 1 ¼ µm diamond suspension and cloth (Arotec, Cotia, Brazil). Part of the block surface was covered with two layers of nail varnish. A Knoop microhardness test was performed with a load of 50 g for 5 s in a hardness tester (HMV 2, Shimadzu Corp., Tokyo, Japan). Five indentations spaced 100 µm apart were made. The blocks had a Knoop hardness number (KHN) mean of 297.35 (SD 16.04).
Initial Roughness Measurement (Ra, µm)
The initial Ra of the enamel blocks was measured using a contact profilometer (Surfcorder SE-1700, Kosaka, Tokyo, Japan). The diamond stylus had a tip radius of 2 µm and readings were performed at a constant velocity of 0.1 mm/s and a load of 0.7 mN. The cut-off value was 0.25 mm and the reading length was 1.25 mm. Five readings were performed on each block and the mean value was calculated. The profilometric readouts were taken from the limit between the area covered with nail varnish and the treatment area.
Following the evaluation of the hardness of the blocks (for the selection of the blocks that were make up the sample) and initial roughness, the duly identified enamel blocks were sterilized in ethylene oxide to avoid infection.
Preparation of Palatal Appliance
Six cavities measuring (5 x 5 x 3 mm) were made in the posterior portion (from premolar to molar) of the acrylic resin palatal appliance (3 on each side). Prior to the placement of an enamel block in a cavity, a drop of sticky wax (Newwax Technew, Rio de Janeiro, Brazil) was placed at the base of the cavity, on which the block was positioned. To maintain the block firm in the cavity, sticky wax was added to the surface of the palatal appliance around the block. The block was placed in the cavity such that its surface remained on level with the acrylic resin of the appliance, allowing uniform contact between the tooth brush/dentifrice and surface of the block and the avoidance of the buildup of bacterial plaque.
Intra and Extra-Oral Phases
The lead-in period was carried out in seven days. During this period and throughout the experimental phase, the volunteers brushed their teeth with the fluoride-free experimental toothpaste. Oral hygiene was performed without the appliances in situ. The palatal appliances were worn in two five-day experimental phases with a two-day washout period. Each day, the appliance was immersed in a 0.2% chlorhexidine aqueous solution for three min before and after use in the mouth to avoid the formation of bacterial plaque. After immersion in chlorhexidine for three min, the appliance was washed and inserted into the oral cavity 1 h prior to the first erosion procedure [7] to allow the formation of the acquired pellicle. Between procedures, the appliance was worn for 2 h to allow saliva to interact with the enamel surface [12]. The blocks were submitted to erosion extra-orally with 25 ml of a cold fresh cola-based soft drink (Coca-Cola®, Vonpar Refrescos S.A., Antônio Carlos, Brazil) four times a day (9:00 am, 11:00 am, 2:00 pm and 4:00 pm) for five min each bath under gentle agitation on a rotary mixer (10 rev/min) (Sandrest, East Sussex, UK) at room temperature. The soft drink used for each erosive procedure was discarded. After two erosion challenges (11:00 am and 4:00 pm), four blocks were brushed either CaCO3 or SiO2-based toothpaste (2 blocks immediately after the erosive challenge and 2 blocks 1 h after the erosive challenge). The other two blocks were not brushed (control). After the abrasion test, the appliance was rinsed for 20 s under running water before reinsertion into the oral cavity. The same procedures were repeated in the cross-over phase. Brushing was performed by a duly trained researcher (MCF) using horizontal movements for 30 s [11], perpendicular to the stylus of the profilometer, with a soft brush (Condor S.A., São Bento do Sul, Brazil) and approximately 0.09 g of toothpaste. The researcher and volunteers were blinded to the toothpaste used in each phase.
The appliance was worn from 8:00 am to 6:00 pm, except during eating, drinking (other than water) or carrying out oral hygiene procedures. At these moments and after 6:00 pm, the appliance was stored in a plastic box and covered with gauze moistened with 0.9% saline solution. After the first experimental phase, new enamel blocks were fixed to the appliance. At the end of each phase, the appliance was immersed in a 0.5% chlorhexidine in a 70% spirit base solution for at least 30 min [13].
The volunteers were instructed to maintain their habitual dietary habits, avoiding acidic beverages and foods, and were instructed to brush the inner surface of the appliance once a day (at night) with a soft brush without toothpaste. The volunteers all live in an area with a fluoridated water supply (mean 0.8 ppm F).
Measurement of Final Roughness (Ra, µm)
The final Ra was assessed in all enamel blocks after experimental periods in the same manner as the initial Ra had been recorded. The change in the roughness was also calculated.
Preparation of Enamel Blocks for Wear Depth in Scanning Electronic Microscope
After the experimental periods, all blocks in each group were analyzed for wear depth using scanning electron microscopy (SEM). Prior to the SEM evaluation, the enamel blocks were immersed in a 3% sodium hypochlorite solution for 24 h to remove the acquired pellicle. The blocks were then rinsed with de-ionized water, air dried and sputter coated with gold. The SEM (Philips XL 30, Philips Electronic Instruments Inc, Mahwah, USA) was operated at 10 to 20 kV, with magnification from 15x to 4000x.
For the analysis of wear depth, the eroded and/or abraded area of the block was fixed perpendicularly to the base of the stub. Five measurements were performed on each block, starting with the surface of the block to the deepest point in the eroded and/or abraded region (Fig. 4).
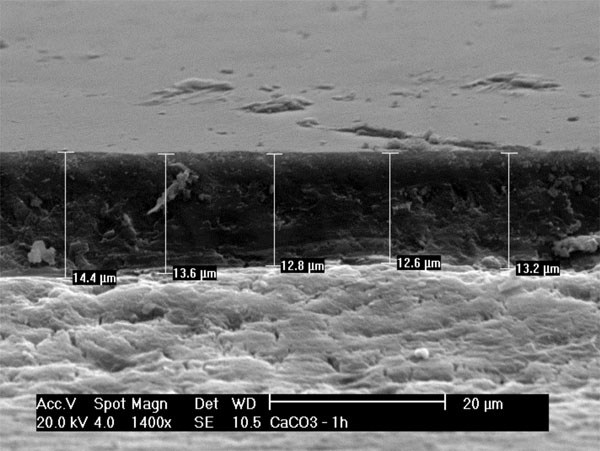
Measurement of wear depth (µm) using SEM.
Statistical Analysis
The blocks were the experimental units. Each group was composed of 24 blocks. The data from the eroded and/or abraded enamel were submitted to the variance homogeneity test (Levene) and normality test (Shapiro-Wilk). The Wilcoxon test was used to compare the initial and final Ra within groups. The Mann-Whitney test was used to compare the change in roughness (final Ra minus initial Ra) between groups. The Mann-Whitney test and non-paired Student’s t-test were used to compare wear depth (µm) between groups. The significance level was set to 5%. All analyses were performed using the Statistical Package for Social Sciences (SPSS for Windows, version 15.0, SPSS Inc, Chicago, USA).
RESULTS
The initial and final Ra data are summarized in Table 1. Table 2 displays the results of the comparison of changes in Ra and wear depth between groups for each toothpaste and between toothpastes. Regarding the change in Ra, significant differences were found between the CaCO3 control and CaCO3 immediate groups (p < 0.001); CaCO3 control and CaCO3 after 1 h groups (p = 0.001); SiO2 control and SiO2 immediate groups (p = 0.014); and SiO2 control and SiO2 after 1 h groups (p = 0.039). Regarding wear depth, significant difference were found between the CaCO3 control and CaCO3 immediate groups (p = 0.001) as well as between CaCO3 control and CaCO3 after 1 h groups (p = 0.022). Regarding wear depth, no significant findings were observed for the SiO2- based toothpaste. Moreover, no significant differences were found in the comparison of dentifrices.
Initial and Final Ra(µm) in Groups
| Groups | Ra | |||
|---|---|---|---|---|
| Initial Median [Range, Interquartile Range] | Final Median [Range, Interquartile Range] | p-value# | ||
| CaCO3 Groups | ||||
| CaCO3 control | 0.07 [0.07, 0.04] | 0.25 [0.52, 0.16] | <0.001 | |
| CaCO3 immediate | 0.06 [0.02, 0.01] | 0.35 [0.45, 0.13] | <0.001 | |
| CaCO3 after 1h | 0.06 [0.02, 0.01] | 0.32 [0.81, 0.11] | <0.001 | |
| SiO2 Groups | ||||
| SiO2 control | 0.08 [0.08, 0.05] | 0.26 [0.65, 0.23] | <0.001 | |
| SiO2 immediate | 0.06 [0.02, 0.01] | 0.36 [0.61, 0.29] | <0.001 | |
| SiO2 after 1h | 0.06 [0.02, 0.01] | 0.34 [0.48, 0.21] | <0.001 | |
24 enamel blocks/group
#Wilcoxon test
Comparison of Change in Raand Wear Depth (µm) Between Groups
| Groups | Change Ra Median# [Range, Interquartile Range] | Wear Depth Median# [Range, Interquartile Range] |
|---|---|---|
| CaCO3 Groups | ||
| CaCO3 control | 0.16 A.,a [0.55, 0.17] | 5.84 A.,a [13.41, 4.77] |
| CaCO3 immediate | 0.30 B.,b[0.46, 0.13] | 9.09 B.,a [8.23, 2.71] |
| CaCO3 after 1h | 0.27 B.,c[0.80, 0.11] | 9.08 B.,a [19.32, 5.65] |
| SiO2 Groups | ||
| SiO2 control | 0.18 A.,a [0.72, 0.24] | 10.45 A.,b [16.14, 6.94] |
| SiO2 immediate | 0.31B.,b [0.62, 0.29] | 8.66 A.,a [15.14, 9.09] |
| SiO2 after 1h | 0.28 B.,c [0.47, 0.21] | 12.4 A.,a [26.16, 8.87] |
24 enamel blocks/group
#Median followed by distinct superscript capital letters show statistically significant difference within each group (CaCO3 group; SiO2 group), and those with lower letters among groups (CaCO3 and SiO2 groups) (p < 0.05).
DISCUSSION
In situ models provide conditions for the determination of dental resistance to acidic and abrasive challenges in the presence of saliva and acquired pellicle. The present study simulated an everyday situation in which the ingestion of acidic beverage four times a day is considered high-frequency consumption and a risk factor for dental erosion [14]. The abrasion assay performed twice reproduces standard oral hygiene habits [15]. The erosion procedures were carried out under gentle agitation to simulate the turbulence that the ingestion of liquid produces on the enamel surface and reproduce its influence on the degree of erosion and demineralization of the enamel [16].
In the comparison between the CaCO3 and SiO2 toothpastes, the eroded/abraded blocks displayed similar behavior both in terms of the change in roughness and wear depth. These results are in agreement with those reported by Turssi et al. [3], who assessed fluoridated toothpastes containing SiO2 and SiO2/CaCO3 and found no significant difference in abrasion of the eroded enamel. It should be stressed that the dentifrices employed in the present study did not contain fluoride, which differs from those used by Turssi et al., [3]. It is likely that the similar behavior between the experimental toothpastes was due to the fact that a softened surface layer is easily removed, regardless of the type of abrasive found in the toothpaste. Hooper et al. [2] also found that fluoridated toothpastes with different relative dentin abrasivity (RDA) values exhibited similar abrasiveness to eroded enamel. According to the authors, the abrasivity of the toothpaste is of minor relevance with regard to the abrasion of eroded enamel. However, different RDA values make a difference on the dentin level, as the study found a significant difference between two toothpastes on this dental tissue, which reflected the difference in the RDA values of the pastes [2]. Another in situ study compared fluoride toothpastes with high and low concentrations of fluoride as well as a non-fluoride toothpaste to a high fluoride toothpaste with the same composition. No significant differences in enamel wear were found between the two fluoridated toothpastes. Although the fluoridated toothpastes (5000 and 1100 ppmF) demonstrated a tendency toward less enamel wear in comparison to the placebo group, the results did not differ significantly from those of the placebo toothpaste. The authors speculated that the dilution of the toothpaste (slurry) and the short time of fluoride application did not allow for the deposition of a CaF2-like material that was able to prevent erosion or abrasion [6]. One would expect better results for fluoridated toothpastes, as found in another study comparing fluoride and non-fluoride toothpastes, in which enamel loss was significantly lesser in the fluoride groups than after brushing with the fluoride-free toothpaste [8].
An in vitro study assessing the enamel abrasivity of fluoridated and non-fluoridated toothpastes with low, medium and high levels of abrasivity found similar results for the fluoridated toothpastes [17]. The lesser enamel surface loss was attributed to the fluoride, confirming the findings of an in situ study in which high amounts of fluoride were capable of reducing the amount of toothbrush abrasion on eroded tooth surfaces [18]. Non-fluoridated toothpastes with a low degree of abrasiveness differed significantly from those with medium and high degrees of abrasiveness, whereas no significant differences were found between the latter two formulas [17], demonstrating that, in the absence of fluoride, there is a tendency toward differences between toothpastes with very different degrees of abrasivity. Another in vitro study evaluating brushing with abrasive silica on the eroded enamel found a non-significant effect on enamel wear. Although brushing with a fluid silica paste caused greater tissue wear, this finding was not significant when compared to brushing with saline solution. These results show that the outer layer of the demineralized enamel is mechanically fragile [19], which is corroborated by the results of the present study as well as a previous study that used ultrasonication to evaluate the amount of removable softened enamel [4].
When analyzing the abrasiveness of an abrasive, one must consider the configuration of the surface, hardness, size, number and distribution of the particles as well as contamination by foreign particles [20]. Although the CaCO3 abrasive does not exhibit uniformity in its crystalline form, which makes it more aggressive to tooth enamel in comparison to the SiO2 abrasive, no significant differences were detected between groups. Thus, it is necessary to consider that glycerin, which is the humectant agent present in toothpastes, tends to decrease abrasiveness, likely due to the lubricating action between the eroded surface and abrasive particles. Evaluating what factors, beyond the abrasive, influence tooth wear caused by brushing on dentin samples, Harte and Manly [21] found that glycerin inhibited abrasion by 88% in comparison to saliva and sodium carboxymethyl cellulose (CMC) as diluents. The authors believe that this result is due to the viscosity of glycerin, which minimizes the abrasive potential to a greater extent than the natural (saliva) or substitute diluent (CMC). This may explain the findings of the present study. It is also necessary to consider that the similar findings between toothpastes were also due to the medium degree of abrasivity of each abrasive, according to the manufacturers.
Significant differences were found in the comparison of the eroded blocks and those in which abrasion was performed immediately or after 1 h of the erosive challenge. This is in agreement with findings reported in the literature [1, 6-8, 22]. As the erosive attack causes the softening of the enamel, this zone is more susceptible to abrasive wear [4].
Although the blocks were randomly distributed among the groups to ensure equivalent distribution, a considerable difference in wear depth was found between the CaCO3 control and SiO2 control groups. The same did not occur between these groups with regard to surface roughness.
Saliva has many properties that offer protection against enamel erosion, such as the buffering of dietary acids, dilution and clearance of erosive agents and the formation of the acquired salivary pellicle, which serves as selective membrane on the enamel surface, providing partial protection against demineralization from microbial acids and erosive attacks [23]. Moreover, saliva can remineralize acid-softened tooth surfaces [24]. Thus, the recommendation of waiting before brushing following an erosive challenge is based on the precipitation of minerals from saliva, which leads to the remineralization of the softened zone. However, studies have demonstrated that the remineralization of the softened zone in the enamel is limited [24, 25]. No significant difference was found between the blocks that were brushed immediately after or 1 h after the erosion challenge. This finding contrasts results described by Jaeggi and Lussi, [7], Attin et al., [1] and Rios et al. [22], who report a significant difference between 0 and 60 min following intra-oral exposure. However, the present results are in agreement with findings described by Ganss et al. [8], who compared immediate brushing with a fluoride-free toothpaste to waiting 2 h before brushing and found no protection of the eroded enamel from greater wear when brushing was delayed. This demonstrates that only limited mineral precipitation occurs on the surface of eroded enamel in the intervals cited, which leads to questioning the viability of waiting one to two hours before brushing, as using a fluoride toothpaste immediately after erosion leads to a significantly greater reduction in the loss of eroded enamel in comparison to using a non-fluoride toothpaste either immediately after or two hours after erosion [8]. Others studies have also failed to demonstrate any significant effect of saliva. In a study assessing changes at the enamel surfaces by surface microhardness measurements, eroded samples exposed to the oral environment for 48 h did not exhibit rehardening [26]. Moreover, samples eroded intraorally by sipping different solutions and retained in the oral environment for three days were not found to exhibit significant rehardening [27].
With regard to abrasivity, the present findings demonstrate that the surface of eroded enamel is mechanically fragile, regardless of the type of abrasive found in non-fluoride toothpaste. Further studies should be carried out comparing fluoride toothpastes with the same concentration of fluoride and different abrasives to confirm or refute the present findings determined with non-fluoride toothpastes and different abrasives. Only one in situ study was found that evaluated dentifrices with the same concentration of fluoride; however, one dentifrice was a silica compound and the other contained calcium carbonate and silica [3]. In the present study, the non-fluoride dentifrices analyzed had a similar composition, with the exception of the abrasive.
This study was limited by ethical matters, such as the ingestion of erosive beverages, and the erosive procedures were therefore performed extra-orally. Thus, the erosive challenge could not stimulate salivary flow and the buffering capacity of saliva. However, this technique and the abrasion procedure, which was also performed extra-orally, allowed greater standardization [28].
CONCLUSION
Surface roughness and wear depth is greater when erosion is combined with dental abrasion, regardless of the abrasive used. Waiting for 1 h to brush the eroded blocks offered no protective effect.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
ACKNOWLEDGEMENTS
The authors thank the volunteers for their participation in this study. This study was supported by Coordination of Higher Education (CAPES), Ministry of Education, Brazil.


