All published articles of this journal are available on ScienceDirect.
Histomorphometric Evaluation of Anorganic Bovine Bone Coverage to Reduce Autogenous Grafts Resorption: Preliminary Results
Abstract
Physiologic resorption due to remodeling processes affects autogenous corticocancellous grafts in the treatment of atrophic jawbone alveolar ridges. Such a situation in the past made overgrafting of the recipient site mandatory to get enough bone support to dental implants in order to perform a prosthetic rehabilitation. Anorganic bovine bone, conventionally used to treat alveolar bone deficiencies in implant surgery, showed a high osteoconductive property thanks to its micro and macrostructure very similar to that of human hydroxyapatite. An original technique provides for the application of a thin layer of anorganic bovine bone granules and a collagen membrane on the top of the corticocancellous onlay bone grafts to reduce in a remarkable way the graft resorption due to remodeling. The results of a clinical prospective study and a histomorphometric analysis done on autogenous grafts harvested from the iliac crest showed that the proposed technique is able to maintain the original bone volume of the corticocancellous blocks.
INTRODUCTION
The successful use of osseointegrated implants in the treatment of partial or complete edentulism requires a sufficient bone support. Bone resorption takes place after tooth extraction, as a consequence of normal turnover process occurring in all bone and is affecting the alveolar ridge either on a vertical or horizontal plan, thus creating an overall reduction of the amount of bone available to dental implants. Whenever rehabilitation in edentulous areas is needed, bone augmentation procedures are available: bony reconstruction provides adjunctive support for implants, allowing prosthetically guided implant placement and improves the aesthetic result of the treatment. Bone grafting of the atrophic sites can be carried out, either prior to implant placement or at the time of implant placement [1, 2].
Bone augmentation using autografts is a reliable technique, as confirmed by several studies. Nevertheless in the first three months after grafting, the volume of the augmented area is diminished.
A variety of surgical techniques have been described to enhance bone volume of deficient implant-recipient sites, such as the use of onlay grafts, ridge splitting or bone condensation. The most common methods include grafting procedures, with or without coverage by a barrier membrane (guided bone regeneration (GBR)). Horizontal ridge augmentation with autogenous block grafts, covered with bioinert expanded polytetrafluoroethylene (ePTFE) membrane, is well documented and results in a good clinical outcome [3, 4].
However, use of ePTFE membranes exhibit some disadvantages: handling and fixation of the hydrophobic membrane is difficult; incision and flap management are demanding, and the technique harbors a certain risk of wound dehiscence with membrane exposure and subsequent site infection [5, 6].
Therefore, clinicians and researchers started looking for alternative barrier membranes in the mid-1990s. Today, after more than 10 years of experimental and clinical experience, the application of bioabsorbable membranes - in particular, collagen membranes - appear to have overcome these problems [7, 8]. However, barrier function and lifespan of resorbable membranes vary considerably and the barrier function is limited to only a few weeks [9, 10]. In contrast, anorganic bovine bone has been shown to resist resorption, after placement into bony defects or as an onlay graft [11]. Therefore, this bone substitute seems to be appropriate to be combined with autogenous bone grafts and collagen membranes, which exhibit only limited barrier function.
Grafting techniques, type of surgery, soft tissue pressure and muscle function, amount of revascularization and some genetic parameters can influence bone resorption [12-16]. Graft overextension is not an appropriate prophylactic measure to limit resorption.
The more the grafted area is over-extended, the greater is the occurring resorption. Resorption also depends on the position of the graft area, for example, the anterior region of the mandible presents a higher rate of resorption compared to the posterior area of the maxilla [16].
In 2005 Maiorana et al. [17] established a procedure to reduce resorption of autogenous corticocancellous bone by using anorganic bovine bone matrix granules (Bio-Oss®, Geistlich Pharma, Wolhusen, Switzerland), spread on top of bone blocks and kept in place by use of porcine collagen membranes (Bio-Gide® Geistlich Pharma,Wolhusen, Switzerland), thereby reducing graft resorption up to 50%.
During Bio-Oss production, the organic scaffold of bovine bone is removed leaving intercrystalline microtunnels and microcapillaries between apatite crystals. The remaining mineral matrix is similar to that of human bone, in terms of chemical composition, morphology and ultrastructure [18, 19].
Aim of this publication is to examine, by histomorphometric evaluation, the resorption – inhibitory effect of anorganic bovine bone, planted onto corticocancellous blocks in a split jaw experiment.
MATERIALS AND METHODOLOGY
The study was performed with 12 adults, with mean age 55 years, affected by class V or VI maxillary atrophy according to Cawood and Howell with a 2 or 3 mm of residual horizontal ridge; the bone hardness of each patients could be associated to the grade 3 – 4 according to the Misch and Judy classification [20, 21] (Figs. 1, 2).

Radiographic representation of an atrophic maxilla class VI Cawood Howell.
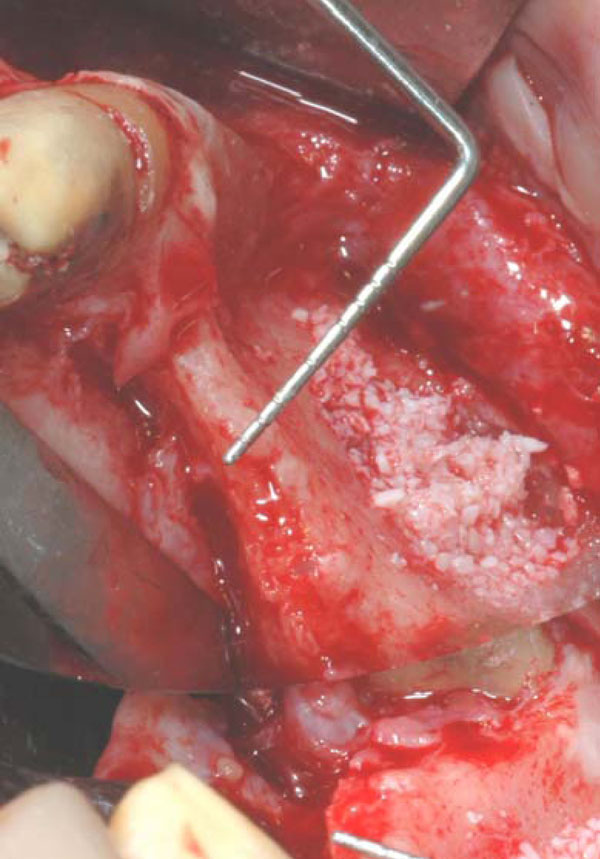
Clinical image of the large bone defect. The probe show how thin is the residual ridge.
The treatment plan provided for onlay corticocancellous grafts harvested from the hip, under general anesthesia (Fig. 3).
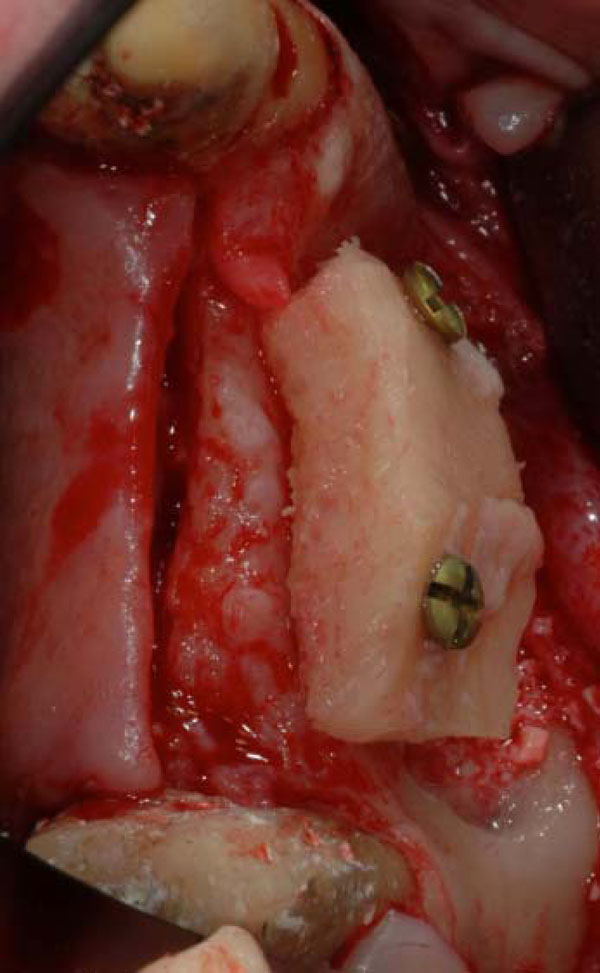
Two screws fixed iliac onlay bone graft for ridge augmentation.
Surgeries were performed in the surgical unit, Policlinico General Hospital Foundation, University of Milan, Italy. Onlay grafts, the grafts were modeled and adapted to the atrophic ridge and secured to the recipient site with transcortical screws (Fig. 4).

Deproteinized bovine bone was then applied to cover the bone graft.
During the intervention, measurements of the alveolar ridge width and height were done prior to graft placement and immediately after augmentation, by means of a periodontal probe.
On top of all onlay grafts, a thin layer of anorganic bovine bone granules was placed covering 50% of the block extension (Fig. 5). The granules were kept in place by a collagen membrane. The other half of each onlay block remained untreated. Implants were placed after a healing period of three months. At the implant positioning stage the new bone ridge was about 8 to 10 mm increased on width.
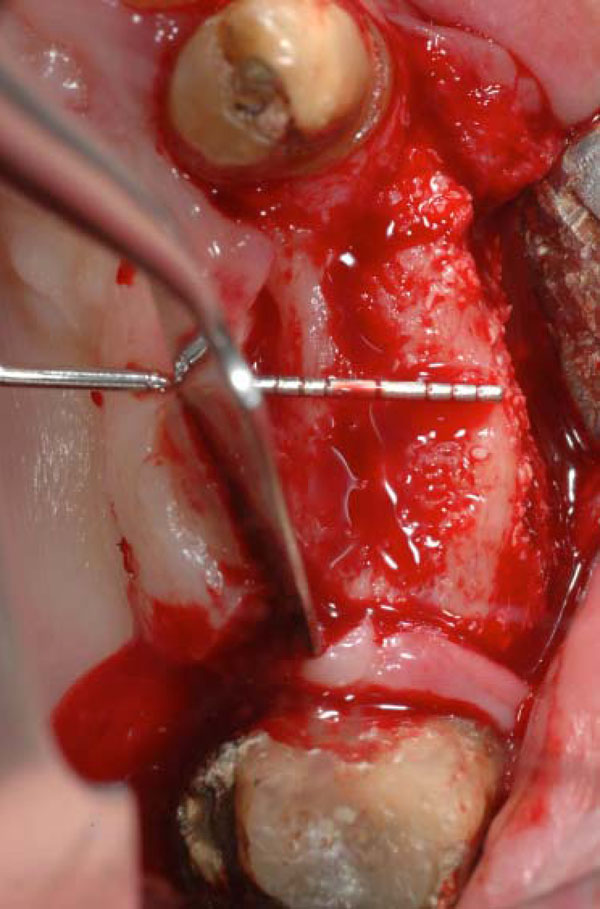
Occlusal view of the ridge at the stage of dental implant placement, 3 months after the surgery. The side test with the BioOss coverage.
Occlusal view of the ridge at the stage of dental implant placement, 3 months after the surgery. The control test without BioOss coverage.
After removing the fixation screws at the time of implantation, bone biopsies were taken at control and test sites with a trephine bur which was moved from buccal to palatal, to get cortical - and the underlying native cancellous bone.
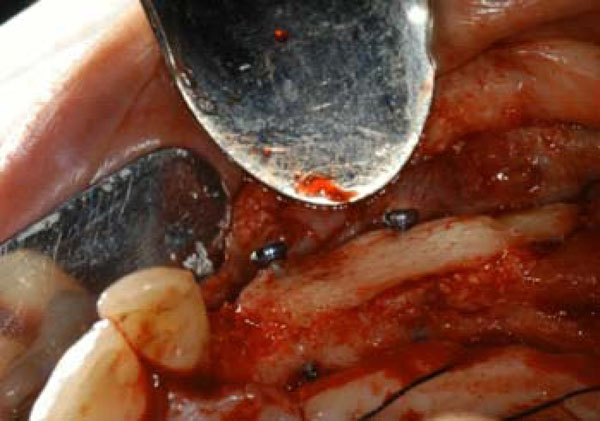
At the time of the second surgery the test site clinically presented the fixation screws totally covered by bone, while the control site showed the head of the screws partially uncovered. Both sites underwent to dental implant positioning and then prosthesis was applied over (Figs. 6, 7).
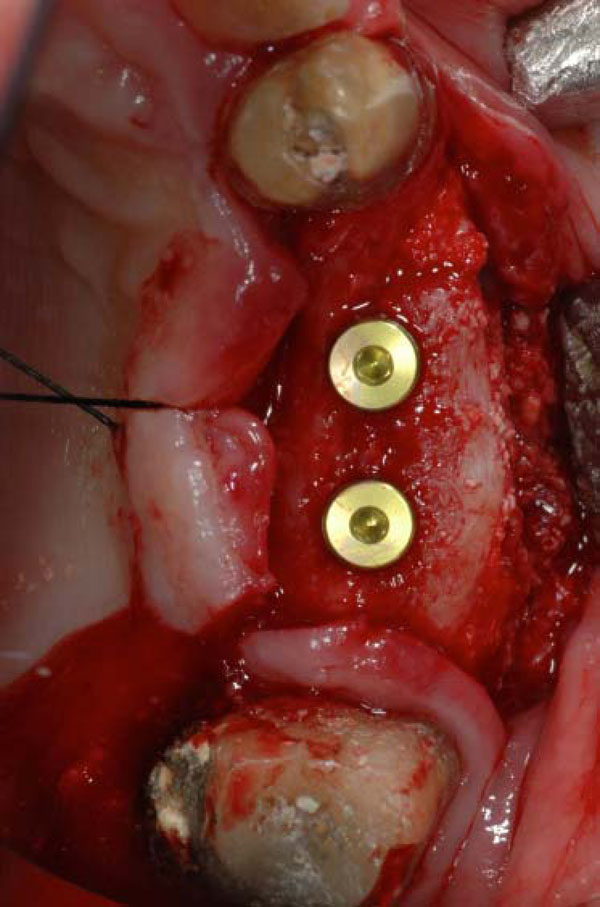
Two dental implants placed on the recovered ridge.
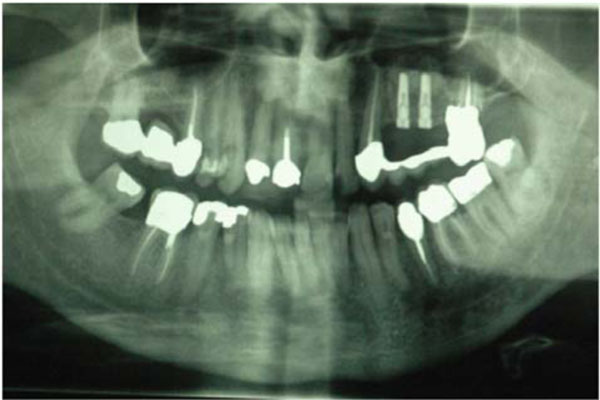
Radiographic control of the two dental implants positioned.
Histology and Histomorphometry
All the histological examinations were perfomed at the Hard Tissue Research Laboratory, Department for Oral- and Maxillofacial Surgery, University Hospital, Freiburg, Germany.
Histologic Preparation
Specimen were fixed in four per cent formaldehyde (Merck, Darmstadt, Germany) for one week and dehydrated using an increasing series of alcohol (70 – 100 % ethanol) for one day with each concentration. Samples were resin-embedded in Technovit 9100 (Heraeus Kulzer, Wehrheim, Germany) according to their specific protocol. After polymerization, specimen were cut in 300 µm sections using a low-speed rotary diamond saw Microslice TM (Metals Research, Cambrige, UK). These sections were mounted onto opaque acrylic-slides (Maertin, Freiburg, Germany) and grounded to a final thickness of approximately 60 µm on a rotating grinding plate (Stuers, Ballerup, Denmark). Specimens were subsequently stained in azure II and pararosaniline (Merck, Darmstadt, Germany).
Histomorphometric Evaluation
Sample imaging was carried out with an Axio Imager M1 microscope equipped with a digital camera AxioCam HRc (Carl Zeiss, Göttingen, Germany). For histomorphometric purpose, the components of interest were digitally labeled. Green was attributed to biomaterial, yellow to older autologous bone and red to newly formed bone. Soft tissue is shown in the original colours of the histological staining procedure. Histomorphometric analysis of sections with Bio-Oss coverage was performed with analySIS FIVE – software (Soft Imaging System, Münster, Germany).
RESULTS
Clinical Evaluation
All the patients had uneventful healing and were later successfully treated with dental implants in the regenerated areas.
Histological Findings
- Specimens without Bio-Oss coverage: Onlay graft without Bio-Oss coverage; Pat. 1 - regio 14
The biopsy (Fig. 8a) shows mature cancellous bone with predominantly lamellar structure. Intertrabecular spaces are filled with connective tissue and bone marrow. Newly formed bone (NB- dark magenta) is added on top of older bone (OB-light magenta). There are no signs of acute inflammatory reactions (original magnification x 50).
Higher magnifications unveil the bone structure, with new woven or lamellar bone deposited on older lamellar bone (Fig. 8b, x 200; 8c, x 630). - Specimens with Bio-Oss coverage Onlay graft with Bio-Oss coverage; Pat. 1- regio 27.
Apart from mature cancellous bone, granules from the Bio-Oss coverage (BO) embedded in newly formed bone are present in the upper part of the biopsy (Fig. 9a).
For histomorphometric purposes (Fig. 9b), the components of interest were digitally labeled. Green was attributed to biomaterial, yellow to autologous older bone and red to newly formed bone. Soft tissue is shown in original colours of the histological staining procedure. A blue line limits the histomorphometric measuring field (original magnification x 50).
Histomorphometric analysis resulted in 19,3% new bone, 1,8% old bone, 26,5% Bio-Oss and 52,4% connective tissue. Therefore, the mineralized tissue fraction amounts to 47,6% in the area of interest.
Higher degrees of magnification show the deposition of newly formed bone (NB) on older bone (OB) and Bio-Oss particles (BO) (Fig. 9c, 9d: x 200). Seams of osteoblasts form Osteoid (OS) and deposit new bone on older lamellar bone (Fig. 9e: x 1000).
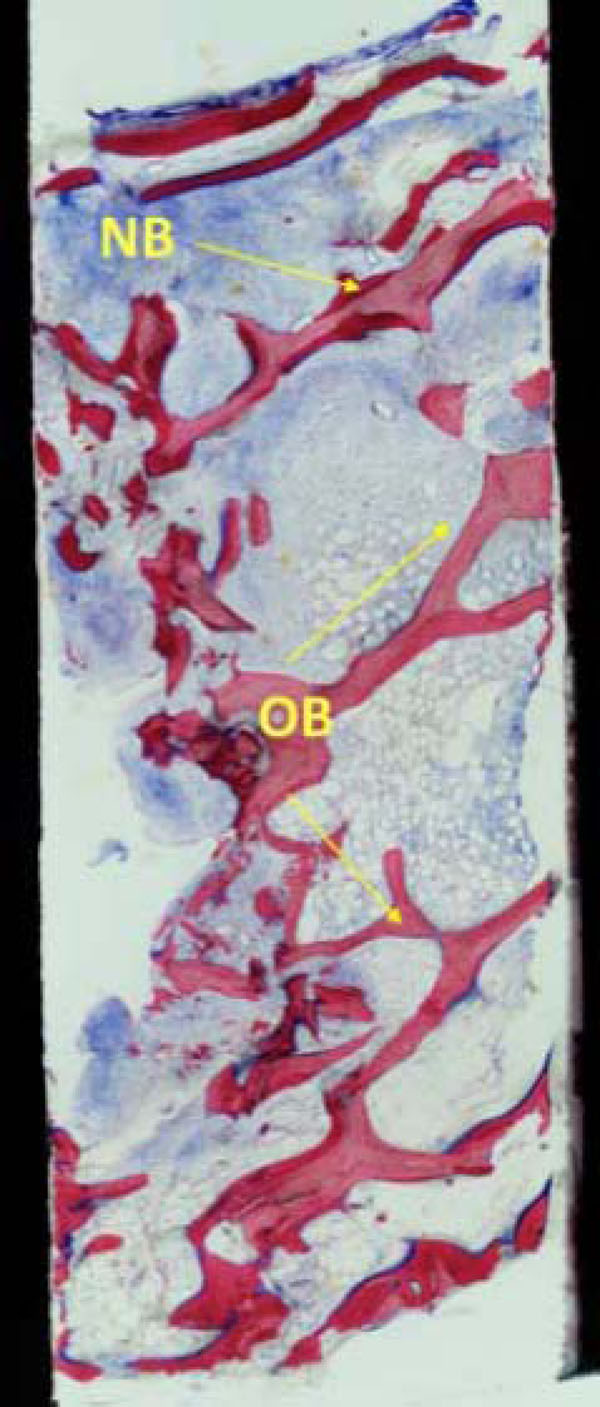
The histological findings show mature cancellous bone with predominantly lamellar structure. There are no signs of acute inflammatory reactions (original magnification x 50).
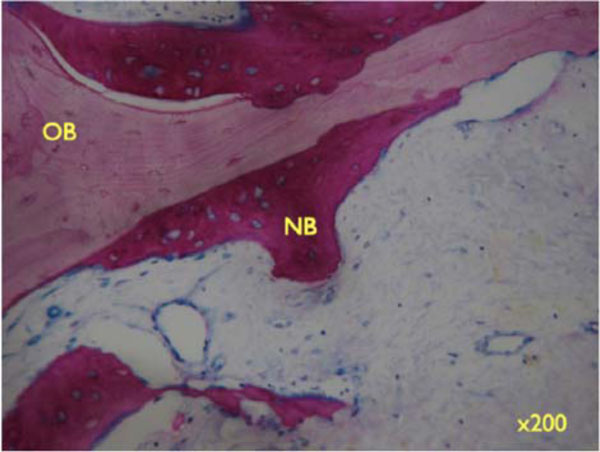
Higher magnifications unveil the bone structure, with new woven or lamellar bone deposited on older lamellar bone (x200).
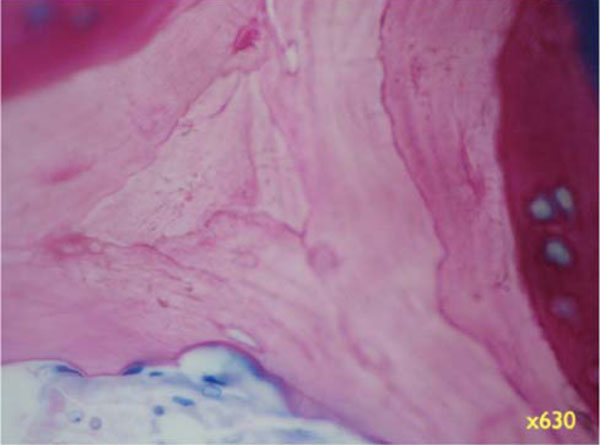
Higher magnifications unveil the bone structure, with new woven or lamellar bone deposited on older lamellar bone (x630).
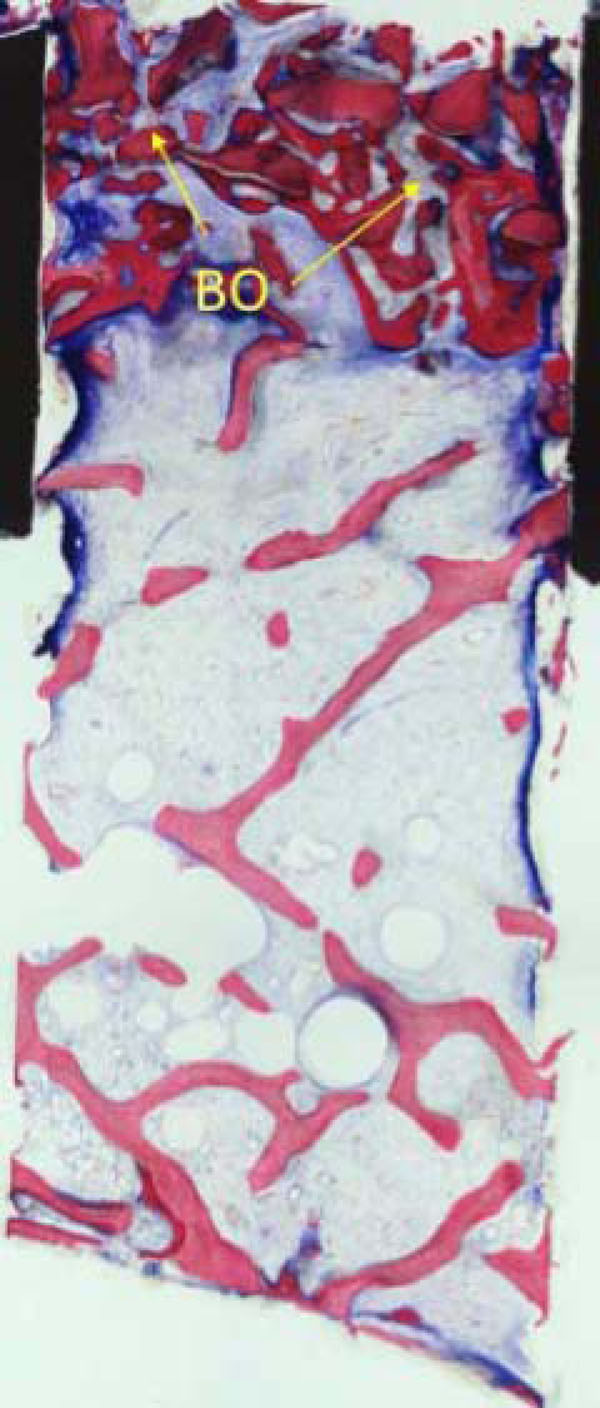
Part from mature cancellous bone, granules from the Bio-Oss coverage (BO) embedded in newly formed bone are present in the upper part of the biopsy.

The components of interest were digitally labelled. Green was attributed to biomaterial, yellow to autologous older bone and red to newly formed bone. Soft tissue is shown in original colours of the histological staining procedure. A blue line limits the histomorphometric measuring field (original magnification x 50).

Higher degrees of magnification show the deposition of newly formed bone (NB) on older bone (OB) and Bio-Oss particles (BO).

Higher degrees of magnification show the deposition of newly formed bone (NB) on older bone (OB) and Bio-Oss particles (BO) (x200).
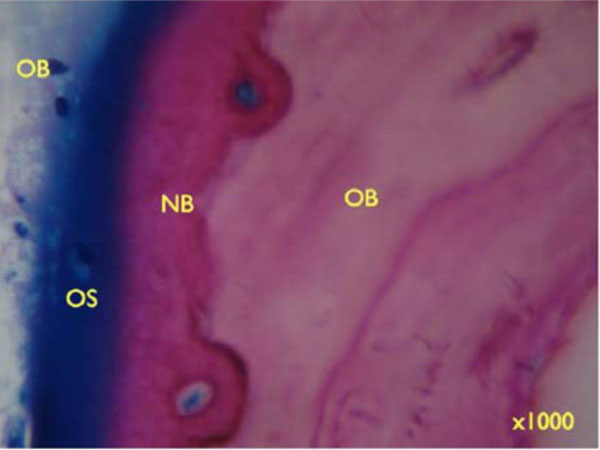
Seams of osteoblasts form Osteoid (OS) and deposit new bone on older lamellar bone (Figure 9e: x 1000).
DISCUSSION
Several studies were undertaken to analyze bone resorption of autogenous block grafts used for oral and maxillofacial surgery. Verhoeven et al. [22] reported that in the first year after bone grafting, resorption is significant and may continue for years. Cortical bone grafts for example, may lose up to 33% of its strength during incorporation, and generally remodels over a 6 to18-month period. Fonseca et al. [23] also reported, that laying corticocancellous bone onto the mandibular buccal cortex for augmentation of the alveolar ridge is a poor method to change ridge morphological structure. Several parameters like embryological origin, architecture, orientation, and graft dimension have been suggested as reasons for the poor volume maintenance of the autogenous bone grafts.
Smith and Abramson [24] proposed two possible mechanisms for calvarial bone graft’s volumetric superiority over iliac grafts. They compared calvarial - to iliac bone grafts using a rabbit model. After 1 year, the calvarial grafts had maintained their size and structure, while iliac grafts had lost at least 75% of their original volume. The authors concluded, that mechanical stress was a key factor for graft survival, believing that iliac bone required mechanical stress to maintain its morphology. After transplantation, loss of this stress led to resorption and poor volume maintenance, when compared to grafts from non-stress-bearing donor sites, such as calvarium. Authors [24] also hypothesized that the microarchitecture of the bone grafts could account for these differences, and noted, that iliac grafts had more cancellous bone than calvarial grafts, and that the increased cancellous component would lead to increased revascularization, resulting in increased resorption compared to calvarial bone graft.
Clinical studies have shown that use of calvarial bone is associated with low post-operative morbidity and a high success rate in bone augmentation procedures [25, 26]. Previous experimental studies indicated, that autogenous grafts with a more cortical microarchitecture, preserve the original bone volume more efficiently over time [27]. These findings suggest that grafts with different microarchitectures exhibit improved patterns of integration into the receptor bed. Comparative experimental studies between onlay grafts, obtained from the iliac crest and the calvarial, have shown that the latter preserves much higher levels of bone volume [28, 29, 30]. However the biomolecular reasons for such effects remain unknown.
Recently, Adeyemo et al. [31] published in 2008 an important microscopical and immunohistochemical study with a sheep model about the healing of onlay mandibular bone graft coverage with collagene membrane or bovine bone substitutes. The study showed that the effect of collagen membrane coverage on bone graft volume maintenance is dependent on membrane stability during healing and Bio-Oss coverage of the bone graft. This was also associated with a remarkable increase in the volume of the augmented bone. These results also suggest that, late induction of apoptosis during development of the mature osteoblast phenotype. A differentiated osteocyte is an end point indicator of the bone remodeling process during bone graft healing.
Bio- Oss action has been reported to promote bone formation by alteration of osteoblast gene expression. Carinci et al. [32] identified genes that are differentially regulated in osteoblasts exposed to Bio-Oss. In an osteoblast-like cell line (MG-63) cultured with Bio-Oss, expression of several genes was significantly changed. These results could explain the reported bio affinity of BioOss for host animals, its biological affinity to osteogenic cells, and its capability to stimulate osteoblastic differentiation.
BioOss remodeling is divided in three different steps: firstly there is an integration with the surrounded bone; then a phase of resorption related to the osteoclasts action and finally a new bone formation when osteoblast cells substitute the BioOss granules with new woven bone [33, 34].
In general, the literature reveals the concerns of clinicians and researchers about the processes involved in the revascularization and maintenance of the volume of autogenous grafts obtained from different donor areas, as well as the influence of the microarchitecture and ossification type on these parameters [35-40].
It is believed, that autogenous bone grafts with a more cortical microarchitecture result in increased maintenance volume. Perforation or decorticalization of the receptor bed - performed by many clinicians – aimed at facilitating graft revascularization, could increase osteogenesis and the graft incorporation rate [41, 42]. However, there are no records in the literature of onlay graft incorporation which correlate the biological events occurring within the graft with variations of volume and density and there are no histological analyses on human about how the graft resorption could be influenced and regulated.
CONCLUSIONS
The results of this investigation show, that the newly applied method led to complete healing and bone filling up to the desired volume. At sites without Bio-Oss coverage, partial exposure of the first threads of the fixation screws was observed, thus indicating that the remodeling process had led to cortical bone resorption. At sites treated with Bio-Oss coverage, no sign of bone resorption was observed, as stated by the absence of exposure of the fixation screws’ threads. This result is in accordance with previous studies from Maiorana and co. [9, 12].
Bone volume maintenance at the test sites can be explained with the action of Bio-Oss, which is a slow - resorbing material, balancing the amount of autogenous bone reduction due to remodeling within the first three months after augmentation with a newly formed bone apposition. The effect of bone maintenance could evidenced by a clinical comparing of the volume of the sites treated with Bio-Oss coverage with the one in the control sites, in which the resorption is stated by the exposure of some threads of the fixation screws, at the moment of implant placement, 3 months after grafting.
Even though this study is presents only preliminary data, the clinical outcomes and the data given by Maiorana and co. in their previous studies, allow to postulate that Bio-Oss coverage technique is a reliable procedure for the volume preservation in onlay graft techniques.
ACKNOWLEDGEMENT
Authors want to thank Annette Lindner for the precious histological work and for her kind and professional cooperation.


