All published articles of this journal are available on ScienceDirect.
In vivo Spectrophotometric Assessment of the Tooth Whitening Effectiveness of Nite White 10% with Amorphous Calcium Phosphate, Potassium Nitrate and Fluoride, Over a 6-month Period
Abstract
To clinically evaluate the effectiveness of Nite White 10% carbamide peroxide with amorphous calcium phosphate, potassium nitrate and fluoride over a 6-month follow-up period. Nite White was applied nightly for 14 days, according to the manufacturer’s instructions. The color of teeth 11 and 21 of twenty one subjects was measured with a spectrophotometer (L*; a*; b*). Subjects were instructed to take note of any tooth sensitivity and gingival irritation. For all three components (L*, a* and b*) statistical significant differences (p<0.05) in the values between base-line (pre-bleaching) and; after treatment, after 1-month, after 3-months as well as after 6-months were found (Wilcoxon Signed Rank Sum Test). Significant differences were also found amongst the ΔE*ab (0-14days) values and; ΔE*ab (0-1 month), ΔE*ab (0-3 months) and ΔE*ab (0-6 months). However, no significant differences were found between pairs of ΔE*ab (0-1 month), ΔE*ab (0-3 months) and ΔE*ab (0-6months). The decrease in ΔE*ab was the highest after 1 month (~30%). The highest decrease in L* was about 58% after 1 month. Over the 14-day treatment period tooth sensitivity was 24.5%. Conclusions: Nite White ACP demonstrated significant tooth-whitening (unit increase = 5.29) with a low tooth sensitivity (25%) probably due to the presence of amorphous calcium phosphate, potassium nitrate, and fluoride. The whitening effect decreased the most after one month and then maintained well even after a 6 month period (units 3.89).
Clinical implications
The product is a good tooth whitener with a color increase of about 5 units accompanied with a low sensitivity.
INTRODUCTION
Vital tooth bleaching has become a common procedure in dentistry to lighten stained or discolored teeth. Different techniques used for tooth-whitening include at-home bleaching (tray-based vital bleaching), in-office bleaching, and over-the-counter whitening products [1]. At-home bleaching using 10% carbamide peroxide is still the most widely used technique because of its relative safety, low-cost and ease of use [2-5].
A common clinical side effect of tooth bleaching is thermal tooth sensitivity and gingival irritation which varies from patient to patient and from product to product [6,7].
Most of the clinical studies evaluated the efficacy of tooth-bleaching using dental shade guides. Although it is a simple method to use, it is not very reliable and is highly subjective [8]. Variables such as observer’s experience, eye fatigue, ambient light conditions and the background against which a tooth is compared may lead to inconsistencies [9]. To overcome these problems colorimetric/spectrophotometric assessment of tooth shade has been recommended [10-13].
Several clinical studies have evaluated the effectiveness and side effects of at-home bleaching products. However, many studies did not apply the suggested manufacturers’ treatment period which would influence the outcome of the results.
Many clinical studies [5,9, 14-18] on various 10% carbamide peroxide products (Nite White Excel, Platinum Professional Tooth-whitening System, Opalescence Whitening Gel, Opalescence PF, Nite White Excel, Nite White Classic) revealed good tooth whitening results which could last for years. However, most of them also reported tooth sensitivity which could be as high as 64% [14].
Over many years, the whitening agent Nite White came through a series of developments (Nite White Excel-2, Nite White Excel-3, Nite White Excel Turbo, etc), until Nite White ACP 10% carbamide peroxide with the patented amorphous calcium phosphate (ACP) was recently introduced. Manufacturers have introduced different compounds such as fluoride, potassium nitrate and amorphous calcium phosphate (ACP) in bleaching products to prevent either hypersensitivity or demineralization effects.
Therefore, the purpose of this study was to evaluate the effectiveness of Nite White ACP 10% carbamide peroxide with ACP over a six month period.
MATERIALS AND METHODS
This study evaluated the effectiveness and side effects of a tray-based home bleaching system Nite White® ACP containing 10% carbamide peroxide, potassium nitrate, amorphous calcium phosphate and fluoride (Discuss Dental, Culver City, CA, USA).
Twenty one volunteers (students, faculty staff) willing to have their teeth whitened were enrolled in the study. Only subjects 18 years of age or above who had sound maxillary incisors, free of caries, restorations, and no crowns with tooth color A2 or darker were included. Subjects with generalized tooth sensitivity, poor oral hygiene, presence of fluorosis or tetracycline staining, on any medical treatment, previous use of bleaching products and pregnant or lactating women were excluded. The study was approved by the Ethics Committee of the University of the Western Cape. All participants included in the study signed an informed consent form after full explanation of the project.
At the initial examination visit, alginate impressions were recorded and models were poured in yellow stone. Study models were trimmed and the maxillary bleaching trays were fabricated from 1 mm soft tray material (Discuss Dental, Culver City, CA, USA) using a vacuum forming technique. The labial surfaces of teeth on models were not blocked for treatment. Trays were trimmed on the labial and lingual surfaces incisal to the free gingival margin, creating a scalloped pattern.
All participants received a prophylaxis with a fluoride containing prophylaxis paste (Nupro Supreme, Dentsply Int, York, PA, USA) to remove the external stains at least two weeks prior to beginning the study. All participants were given verbal and written instructions about the use of the material. The bleaching material (Nite White ACP 10% carbamide peroxide) was administered overnight for 14 days using the customized bleaching trays. Participants were instructed to brush twice a day with toothpaste provided to standardize the fluoride levels and oral hygiene. The complete treatment process was according to the manufacturer’s instructions.
A sensitivity sheet was given to all participants to record any tooth sensitivity experienced during the 14-day treatment period in one of five categories: 1- none, 2-mild, 3- moderate, 4- considerable, 5- severe.
Objective tooth color measurements of maxillary central incisors were taken using a spectrophotometer (CM-2600d, Konica Minolta Sensing, Inc., Japan) with a 6 mm diameter probe. The spectrophotometer (Konica Minolta Sensing, Inc., Japan) measured the color of teeth based on CIE L*a*b* color space system defined by the International Commission on Illumination [19]. Before use, the spectrophotometer was calibrated as outlined by the manufacturer. The color of an area (6 mm) at the centre of the crown of each maxillary central incisor (11 and 21) was measured three times with the spectrophotometer. The average of three measurements was considered as the measured value. The measurements were performed after the prophylaxis prior to bleaching (baseline), after 14 days of treatment (end of active bleaching period), as well as after 1 month, 3-months and 6-months.
Differences in individual color components (ΔL*, Δa* and Δb*) at the mentioned different time intervals were calculated, while total color differences were measured using the formula: ΔE*ab [19].
Data were analyzed using the Wilcoxon Signed Rank Sum Test on a significance level of 5%.
RESULTS
Before treatment (at base-line), no significant differences were found in all three different components (L*, a* and b*) between teeth 11 and 21 (Wilcoxon Signed Rank Sum Test). Therefore, the values for teeth 11 and 21 were subsequently pooled for any further analysis. For all three components (L*, a* and b*) statistical significant differences (p<0.05) in the values (Figs. 1-3) between base-line (pre-treatment), and after treatment (14 days later), after 1 month, after 3 months as well as after 6 months were found (Wilcoxon Signed Rank Sum Test). For all three components, significant differences (decrease) were found between just after treatment and 1 month, 3 months as well as after 6 months (Wilcoxon Signed Rank Sum Test). However, no significant differences were found in the median values of the three components amongst a 1-month, 3-months and 6-months period.
Significant differences were found in the median ΔE*ab (0-14days) values and ΔE*ab (0-30days), ΔE*ab (0-1month), ΔE*ab (0-3 months) and ΔE*ab (0-6 months). However, no significant differences were found between pairs of ΔE*ab (0-30days), ΔE*ab (0-3 months) and ΔE*ab (0-6months). Although there was a decrease in ΔE*ab from after treatment to 1 month (30%), to 3 months (37%) and to 6 months (28%), it was not statistically significant (Fig. 4). The median decrease in ΔL* from after treatment was about 36% after 1 month with a further decrease to ~46% after 3 months where it more or less stabilized for at least 6 months (Fig. 1). The median a* value decreased over time from ~12% after 1 month to 33% after 3 months and ~35% after 6 months (Fig. 2). The median b* values also decreased, with ~29% after 1 month, ~41% after 3 months and ~42% after 6 months (Fig. 3).

The 25 percentile, median and 75 percentile differences in the L* values between the base-line (before treatment) and after treatment (14 days), after 1-month, 3-months and after 6-months.
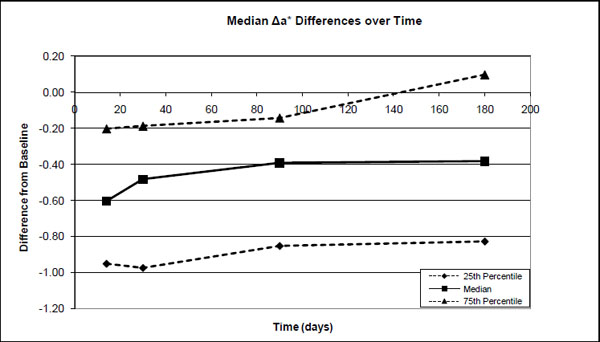
The 25 percentile, median and 75 percentile differences in the a* values between the base-line (before treatment) and after treatment (14 days), after 1-month, 3-months and after 6-months.
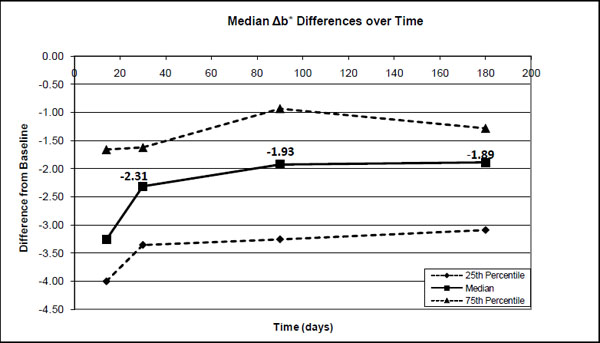
The 25 percentile, median and 75 percentile differences in the b* values between the base-line (before treatment) and after treatment (14 days), after 1-month, 3-months and after 6-months.

The 25 percentile, median and 75 percentile differences in the ΔE*ab values between the base-line (before treatment) and after treatment (14 days), after 1-month, 3-months and after 6-months.
Of the 21 subjects, 12 (57%) experienced tooth sensitivity at some point during the active bleaching period of 14 days. The majority of the subjects intermittently experienced only mild sensitivity up to 6 days or less with an average duration of 38 minutes per day. Only 4 subjects experienced sensitivity for more than 9 days with moderate to severe sensitivity of short duration (10 minutes) for 2 to 3 days. The duration of sensitivity was more in the first week of bleaching than in the second week.
Of the total of 294 days of bleaching for the 21 subjects (14 days per subject) there was 72 days (24.5%) of sensitivity. The sensitivity was mild for 60 days (20%), moderate to severe for 12 days (4%).
DISCUSSION
In this study a relatively sophisticated spectrophotometer was used to determine the color change of teeth. Less sophisticated spectrophotometers or colorimeters tend to have a high deviation between measurements and the results should be treated with caution. Apart from being a small computer the spectrophotometer used in this study (Model: CM-2600d, Konica Minolta Sensing, Inc., Japan) has its own built-in light source, constant illumination/viewing angles and constant “observer”, which means observer conditions are uniform for all measurements. Before use, the instrument was calibrated, as outlined by the manufacturers. Briefly, a zero calibration into the air was first done, followed by a white calibration on the supplied white calibration plate. The instrument (Model: CM-2600d, Konica Minolta Sensing, Inc., Japan) has diffused illumination with a viewing angle of 8-degrees. The repeatability in terms of the standard deviation when ∆E*ab is measured is within 0.04, while the inter instrument agreement for the ∆E*ab measurement is within 0.2 (MAV/SCI).
In this study, the CIE system of color [20] was used which is a mixture of hue (green, red, blue, yellow etc), lightness (bright colors and dark colors) and saturation (vivid colors and dull colors). The instrument numerically measures the quantity of color in a three dimensional color space (L*a*b*). Where L* indicates lightness/darkness (white/black), the a* value varies from a negative side (more greenish) to the positive side (more reddish), while the b* value varies from the more blue side (negative side) to the more yellow side (positive side).
Many studies used shade guides to measure the color of teeth. However, the shade guide has many limitations of which the major ones are: it is observer sensitive and it only gives an overall color value which is not broken down into L*, a* and b* components, as for the spectrophotometer. Furthermore, it was reported that intra-evaluator agreement for the shade guide can be as low as 60% [21].
It is also possible to measure the overall color change (ΔE*ab) with the spectrophotometer as one value (like the shade guide). The formula then is: ΔE*ab = [(ΔL*)2 + (Δa*)2 + (Δb*)2]1/2, where ∆L*, ∆a* and ∆b* are the changes which occurred in these components [19]. However, measuring only ΔE*ab would limit the information about the real color of the tooth or the real change in color which took place during a treatment with a whitener which might vary from between different products.
The area between the 25 percentile and 50 percentile (Figs. 1-4) gives an indication where 50% of the values are. This spreading of the results can be expected as a selection of subjects were made on the basis of teeth color A2 or darker and it can be expected that the lighter the teeth to be bleached the less the change would be and vice versa.
As in this study, most studies [2, 9, 22-24] also found relatively small changes in the a* values and the major changes during tooth whitening took place in the L* and b* values.
From Fig. (4) it can be seen that the major ΔE*ab decrease of ~32% took place during the first month and then became more or less stable for at least 6 months. The decrease after 1 month (which is 2 weeks after the 14 day treatment) was already 32% which showed that the decrease just after treatment is quite fast. However, there was still about a 70% improvement which lasted for at least 6 months. On the other hand the decrease in the whiteness/brightness (L*) and the yellowness (b*) were approximately 46% and 42%, respectively. The a* value also decreased fast, but least (Fig. 2) and stabilized at a decrease of ~35%. The question however is whether this decrease of 46% is still acceptable as far as the whiteness/brightness of teeth is concerned or will it be necessary to re-bleach after 6 months. The answer to this question will vary from person to person. For some it might not be acceptable and vice versa.
No clinical trials could be found for Nite White 10% ACP. However, for the other previous Nite White products it varied from a total unit color improvement of 2.5 [25]; 6.0 [25]; 8.3 [26]; 8.81 [27] up to 9.6 units [9] in comparison to the 5.3 found (Fig. 4) in this study. The improvement as a result of the Nite White ACP treatment was over 4 units in the L* value, 3.3 units in the b* value and only 0.6 units in the a* value (Figs. 1-3).
In another spectrophotometric study [9] approximately the same distribution was reported (ΔE*ab) for 10% carbamide peroxide (Nite White Excel, treated for 4 hours daily for 14 days and Opalescence 10% PF, treated for 4 hours daily for 14 days) with values 3.89 and 3.77, respectively. They reported a significant change of more than 3.6 in ΔE*ab (for both Opalescence 10% PF and Nite White Excel) which was lower than the 5.25 found for Nite White ACP (treated overnight for 14 days) in this study. Furthermore, a ΔE*ab increase of 3.66 units [17] (overnight for 14 days) and 3 units [3] (overnight for 14 days) was also reported for Opalescence 10% PF.
In contrast to this study, many studies did not apply the manufacturer’s recommendation for the treatment period (overnight for 14 days). It also seems that the treatment period has an effect on the results and that a longer period of treatment and stronger peroxide solution is associated with better whitening [2-5].
Tooth sensitivity is a common clinical side effect of tooth bleaching and is expected to vary with: the treatment period, peroxide concentration, and type of bleaching agent. Therefore, it is difficult to compare tooth sensitivity results reported in the literature with our results. However, Karpinia et al. [18] reported tooth sensitivity or gingival irritation for 35-40% of the subjects who used Nite White Excel 2 (10% carbamide peroxide) for 2 hours per day for 14 days. Leonard, et al. [16] reported tooth sensitivity or gingival irritation for 66% of the patients who bleached their teeth with Nite White Classic (10% carbamide peroxide) during the active treatment period. On the other hand, Callan, et al. [25] reported for Nite White Excel 2Z (2 week treatment period) only a 16% sensitivity but that was over a 302 days period. This can be expected as it was also found in this study that the sensitivity disappeared at the end of the treatment period (14 days). Pohjola et al. [15] reported a 25% gingival sensitivity for Nite White Excel 2Z when treated for 83 days but did not report sensitivity for any one of the products. Again it was calculated over a long period and what really happened over the treatment period would be masked. Thus, these values are well removed from the 25% found for Nite White 10% carbamide peroxide (with amorphous calcium phosphate, potassium nitrate, and fluoride) in the present study during the 14 day treatment period.
Some believe that tooth sensitivity can be decreased by the addition of various chemicals such as potassium nitrate, fluoride and amorphous calcium phosphate (ACP) [7]. In this sense a significant reduction in the hypersensitivity was reported with the addition of amorphous calcium phosphate (ACP) to a 16% carbamide whitener [28]. However, in another tooth whitening study it was found that the presence of amorphous calcium phosphate relative to the presence of potassium nitrate and fluoride could not make a difference in the sensitivity over a three month period [6]. Matis et al. [6], compared two bleaching products with different desensitizing agents and reported that 15% carbamide peroxide with potassium nitrate and fluoride showed no significant difference in sensitivity compared to 16% carbamide peroxide with amorphous calcium phosphate. Tam [29] reported that the addition of potassium nitrate and fluoride to 10% carbamide peroxide bleaching gel resulted in less sensitivity compared to the bleaching gel without any desensitizing agent.
The above clinical data indicated no difference in tooth sensitivity between amorphous calcium phosphate on the one side and potassium nitrate plus fluoride on the other side. However, it is possible that a combination of all three might have a combined effect. A deduction which could be made from the above is that Nite White 10% ACP (with amorphous calcium phosphate, potassium nitrate, and fluoride) with all three mentioned chemicals showed a lower tooth sensitivity (25%) than its predecessors [5, 9, 14-18] without it.
The final question then would be whether the total color change found in this study would be visible to the human eye. Tooth colour is a combination of intrinsic and extrinsic colourations [30] and follows highly irregular light paths through the tooth structure before emerging at the surface and reaching the eye of the observer [31]. In and article [32] it was reported that only a change in the ΔE* value of about 3.3 and more would be visible by the human eye. The change in ΔE* (5.29) found in this study (Fig. 4) therefore, is far more than the value mentioned and it can be accepted that the use of Nite White 10% as a tooth whitener should also have a clearly visible tooth color improvement.
CONCLUSION
Nite White ACP demonstrated significant tooth-whitening (unit increase = 5.29) with a low tooth sensitivity (25%) probably due to the presence of amorphous calcium phosphate, potassium nitrate, and fluoride. The whitening effect decreased the most after one month and then maintained well even at a 6-month period (units 3.89). The whiteness/brightness (L*) decreased with 36%, the a* value with 12% and the b* value (yellowness) with 29% after 1 month.


