All published articles of this journal are available on ScienceDirect.
The Relationships between Two Different Drinking Water Fluoride Levels, Dental Fluorosis and Bone Mineral Density of Children
Abstract
This field study included the whole population of children aged 10–15 years (77 from a 0.19 mg/L F area; 89 from a 3.00 mg/L F area), with similar nutritional, dietary habits and similar ethnic and socioeconomic status. The fluoride concentration in the drinking water, the bone mineral content, the bone density and the degree of dental fluorosis were determined. The left radius was measured for bone width, bone mineral content, and bone mineral density. The mean fluorosis score was 1.3 in the low fluoride area and 3,6 in the high fluoride area. More than half the children in the low fluoride area had no fluorosis (scores 0 and 1) while only 5% in the high fluoride area had none. Severe fluorosis (30%) was only observed in the high fluoride area. The Wilcoxon Rank Sum Test indicated that fluorosis levels differed significantly (p < 0.05) between the two areas. No relationships were found between dental fluorosis and bone width or between fluorosis and bone mineral density in the two areas (Spearment Rank correlations). A significant increase in bone width was found with age but no differences amongst and boys and girls. A significant positive correlation was found in the high fluoride area between bone mineral density over age. In the 12-13 and 13-14 year age groups in the high fluoride area, girls had higher bone mineral densities. However, a significant negative correlation (p<0.02) was found for the low fluoride area (0.19 mg/L F) over age.
INTRODUCTION
Today it is believed that fluoridated water (whether naturally or artificially fluoridated) can be generally regarded as a safe, simple and cost-effective public health measure to prevent dental caries and has its greatest influence on socially disadvantaged children who have the most tooth decay [1-6]. However, a too high drinking water fluoride concentration will lead to unesthetical dental fluorosis and the development of skeletal fluorosis. In general, dental fluorosis increased with increasing drinking water fluoride concentrations [7-12]. In 2005, the American Dietetic Association (ADA) [13] reaffirmed the importance of appropriate fluoride as an important element for all mineralized tissues in the human body. They stated that topical and systemic fluorides have resulted in major reductions in dental caries, while water fluoridation is considered as one of the most beneficial public health measures throughout the life span.
Bone strength is primarily determined by bone mineral density (BMD), but bone quality (e.g. bone remodeling, structural and material properties) is also an important determinant of bone strength. It was also suggested that optimal drinking water fluoridation (1 mg/L), did not influence peak bone density [14].
A summary of 33 studies since 1991 was done by the National Health and MRC (medical research council) of Australia [15] on the effects of fluoride on bone. One of two cohort studies showed an increase in fracture incidence at fluoride levels four times greater than optimal water fluoridation and the other showed no effect after 20 years’ optimal fluoridation. The results of ecological studies were found to be conflicting but the clinical trials predominantly showed increased bone density with fluoride treatments of 9-23 mg F per day for 4 years. A low risk of hip fracture for people ingesting drinking water containing 1 mg/L F was also reported [9]. From the above studies it was concluded [8] that fluoride up to 1 mg/L does not have an adverse effect on bone strength, bone mineral density or incidence of fracture. A report by the National Research Council [16] concluded that the EPA’s (environmental protection agency) maximum drinking water fluoride standard (4 mg/L) is too high and does not protect against adverse health effects such as skeletal fluorosis especially for life-long residents in these areas. However, the level for aesthetic or cosmetic effects such as dental fluorosis was set lower at 2 mg/L fluoride [16].
In a low fluoride area with a high dental fluorosis index of 3.7 which was attributed to high fluoride containing brick-tea, skeletal abnormalities in the wrist were associated with the early-stage of skeletal fluorosis [17]. The question then arises whether low drinking water fluoride concentrations which were normally associated with lower degrees of dental fluorosis have any effect on bone density and whether different degrees of fluorosis scores can be used as a possible indication of bone density and possible warning signs for skeletal fluorosis.
Therefore, the purpose of this study was to determine relationships amongst two different drinking water fluoride levels, dental fluorosis and bone mineral density of children.
MATERIALS AND METHODOLOGY
The study included all available children aged 10–15 yrs, with similar nutritional and dietary habits and similar ethnic and socio-economic status, from a low fluoride area (0.19mg/L F) and a high fluoride area (3.00 mg/L F). Both areas are located in arid rural sections of South Africa and during this study they were dependent on boreholes for their drinking water. The number of children investigated were 77 (boys = 42) from the low F area and 89 (boys = 42) from the high F area. Every effort was made to ensure that the children had been born and raised in the areas. The children were examined for dental fluorosis using Dean’s criteria according to the World Health Organization guidelines [18]. Water fluoride levels were determined potentiometrically, according to the method described by Nicholson and Duff [19] and were analyzed over a period of approximately 10 years [20,21].
The examiner was standardized and calibrated for examiner variability, using an expert (gold standard) prior to and during examinations [22]. Examiner agreement (inter- and intra-) was determined using the weighted kappa, which takes into account the relative impact of each possible disagreement. Linear weights, which are proportional to the deviation of individual ratings, were used [23]. The intra- and inter-examiner agreement scores for the fluorosis index (k = 0.78 and 0.70, respectively) were substantial according to the scale of Landis and Koch, thus meeting the scientific requirement for validity and reliability [24]. Agreement was also monitored throughout the study by re-examining 10% of the sample, with the result being of the same order as the pre-survey finding.
The Norland single energy (125I) photon absorptiometer (Model 278A) was used for the bone measurements [25, 26]. The left radius was measured for bone width and bone mineral content. The exact point of measurement of the radial bone mass was determined by measuring the distances between the proximal end of the olecranon and the distal tip of the ulna styloid, dividing the measurement distance by 3 and then positioning the support plate so that the beam passed through the point at this 1/3 distance from the ulna styloid. The arm was secured with straps and the subjects cautioned not to move during the readings. The bone width (BW) and bone mineral content (BMC) values were measured (at this site it is mainly cortical bone) and the bone mineral density (BMD) (normalised for bone width) calculated. Three readings were taken from each subject and the mean values were recorded in the data basis.
To accommodate concerns regarding the difference in onset of adolescence the genders were separated into three age groups, which resulted in three age groups and six gender groups.
RESULTS
Since analysis of the data (Mann-Whitney U test) showed no significant difference in the fluorosis scores between girls and boys, the results for the these two series were combined in the fluorosis data. The prevalence (Table 1) of fluorosis (scores 2-5) was 49% in the low fluoride area and 96% in the high fluoride area. There was 38% children in the low fluoride area with no fluorosis (Table 1) in contrast to only 1% in the high fluoride area. No children in the low F area had severe fluorosis in comparison to 30% in the high F area. The mean fluorosis score (1.3) was significantly lower (Wilcoxon Rank Sum Test) in the low F area than in the high F area (3.6).
Dental Fluorosis Score by Fluoride Area
* standard deviation.
Table 2 summarizes the mean values (standard deviation in brackets) over age for bone width, bone mineral content and bone mineral density for the two different areas.
The Mean Values for Age (Years), Bone width (cm), Bone Mineral Content (g/cm) and Bone Mineral Density (g/cm2) for the Two Different Areas
| Age Groups | 10 & 11 | 12 & 13 | 14 & 15 |
|---|---|---|---|
| Average Bone Width (cm) | |||
| High F area girls | 0.97 | 1.09 | 1.10 |
| Sample number (n) | 23 | 13 | 11 |
| High F area boys | 0.98 | 1.08 | 1.14 |
| Sample number (n) | 19 | 14 | 9 |
| Low F area girls | 0.98 | 1.04 | 1.18 |
| Sample number (n) | 14 | 18 | 3 |
| Low F area boys | 1.01 | 1.15 | 1.21 |
| Sample number (n) | 13 | 20 | 9 |
| Average Bone Mineral Content (g/cm) | |||
| Age groups | 10 & 11 | 12 & 13 | 14 & 15 |
| High F area girls | 1.29 | 1.56 | 1.80 |
| High F area boys | 1.29 | 1.41 | 1.80 |
| Low F area girls | 1.26 | 1.33 | 1.18 |
| Low F area boys | 1.32 | 1.29 | 1.52 |
| Average Bone Mineral Density (g/cm2) | |||
| Age groups | 10 & 11 | 12 & 13 | 14 & 15 |
| High F area girls | 1.34 | 1.51 | 1.63 |
| High F area boys | 1.35 | 1.31 | 1.58 |
| Low F area girls | 1.29 | 1.27 | 1.00 |
| Low F area boys | 1.31 | 1.12 | 1.22 |
Fig. (1) shows the bone width (BW); (Fig. 2) the bone mineral content (BMC) and (Fig. 3) the bone mineral density (BMD) for boys and girls over the 3 age groups for the low (0.19 mg/L F) and high fluoride (3.00 mg/L F) areas. The values encircled were found to be not statistically significantly different within a particular age group (p > 0.05; Tukey-Kramer Multiple-Comparison Test).
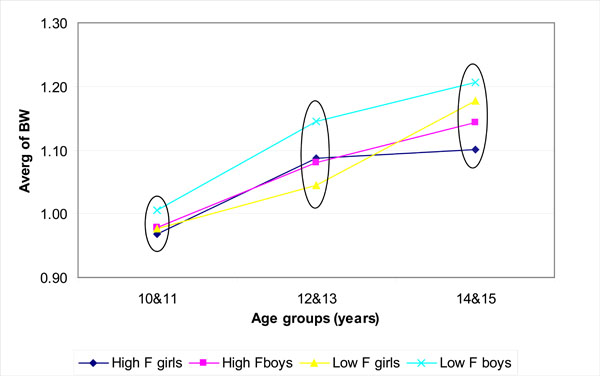
The mean values of the bone width (BW) over years for the low F areas (0.19 mg/L F) and high F area (3.00 mg/L F). The values encircled (within each age group) were not significantly different (p > 0.05; Tukey Kramer).
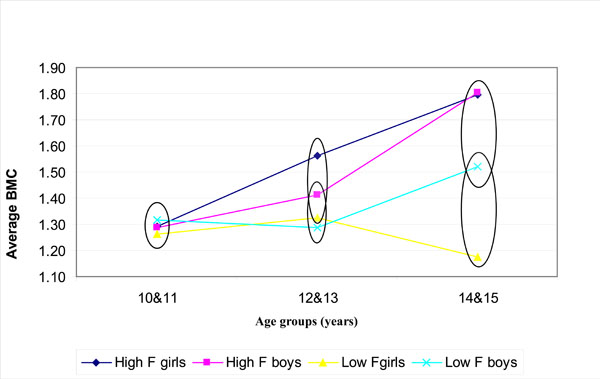
The mean values of the bone mineral content (BMC) over years for the low F area (0.19 mg F/L) and high F area (3.00 mg F/L). The values encircled (within each age group) were not significantly different (p > 0.05; Tukey Kramer).
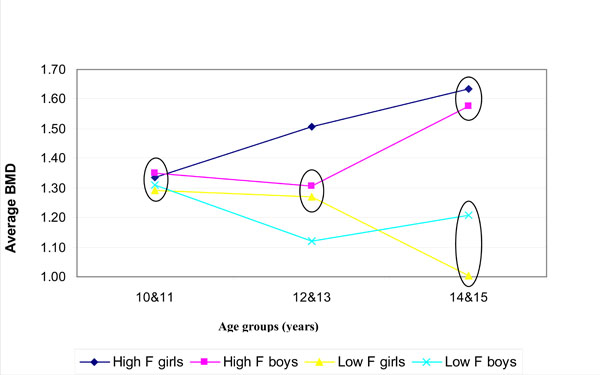
The average values of the bone mineral density (BMD) over years for the low (0.19 mg/L F) and high (3.00 mg/L F) F areas. The values encircled (within each age group) were not significantly different (p > 0.05; Tukey Kramer).
No relationship was found between dental fluorosis and bone density in any of the two areas (Spearman Rank correlation). However, statistical significant differences (p<0.05) were found in the fluorosis levels between the two different areas (Wilcoxon Rank Sum Test).
In general boys had wider bone than girls (Fig. 1). However, there was no significant difference in bone width within boys or girls or between boys and girls in any of the age groups (6 groups; Fig. 1). A strong positive linear relationship was detected between age and BW within each of the genders and two fluoride groups (p<0.05; Spearman rank correlation).
The BMC increased with age in the high fluoride area for boys and girls but varied in the low fluoride area. Significant differences in the BMC in the 14-15 year age group and 12-13 year age group (Fig. 2) was found amongst boys from the high fluoride area (3 mg/L) and girls from the low fluoride area but no differences in the 10-11 year age group.
In general, the BMD (Fig. 3) increased with age in the high fluoride area but decreased significantly with age in the low fluoride area. (p< 0.05; Spearman Rank Correlation Test). In a comparison between the two different fluoride areas for the bone mineral density at a specific age group, differences were found (Fig. 3; p < 0.05; Tukey Kramer Multiple Comparison test). In the 10-11 year age group no significant differences were found, while in the 12-13 year age group there was a significant difference between girls from the high fluoride area and boys from the low fluoride area. In the 14-15 year age group the BMD of boys and girls in the high fluoride area differed significantly from the boys and girls in the low fluoride group.
DISCUSSION AND CONCLUSION
This study was conducted in two groups of children who lived continuously since birth in separate areas, each of which had constant levels of fluoride in the drinking water. In most cases, not only had the children lived in a specific area since birth but so had their parents. The children in the two fluoride areas were of mixed ethnicity, originating from Khoi, Caucasian, and Negroid roots that developed into a homogenous ethnic group over many years. The children were also from the same low socio-economic group, as identified according to the mean values of the residential properties and/or units status as described and used by Du Plessis [6] for South Africa. The staple diet throughout consisted mainly of bread and potatoes with sporadic intake of other vegetables and meat. Personal communication with primary health care personnel did not reveal the prevalence of obvious under-nutrition, nor could any dietary habits be detected that might have contributed significantly to the ingestion of fluoride. These children had virtually no dental care or fluoride therapy, including the use of fluoride-containing toothpaste, prior to this study. Furthermore, the drinking water sources (bore holes) did not change over the life-span of the children, which is an advantage that should be appreciated. Thus, only small insignificant seasonal variations [27] in the drinking water fluoride levels could have occurred. The average maximum daily temperature for the two areas was also high (~25 ºC) and the rainfall very low. Therefore, one would expect elevated water consumption [28].
In areas in Nigeria [29] where the water F levels varied between 0.05 and 0.4 mg/L it was stated that 49% had no fluorosis and 42% very mild which is about similar to our findings (Table 1) in the low F area (F=0.19 mg/L). Also quite similar to our findings, a fluorosis index of 1.3 was reported for a low F area (F=0.25 mg/L) in the Sudan [11] but a value of 2.1 was reported for an area with 2.5 mg/L F in the water in comparison to 3.6 in our 3.0 mg/L F area. In an area (F=2.15 mg/L) in Kenia [12] dental fluorosis was found to be 100% with 50% of the children with severe fluorosis (score 5), which is worse than in our high F area (30%; Table 1). A very high fluorosis value (4.5) was also reported in South Africa [8] for a drinking water F level of 3.7 mg/L for life-long resident children. In a survey conducted in two high-fluoride areas (3.5 and 12.5 mg/L) in Ethiopia [30], it was found that all children born there had fluorosis. However, in a low drinking water F area a dental fluorosis score of 84% was also reported but attributed to brick-tea drinking [17] and associated with skeletal abnormalities in the wrist. It was further suggested that the observed dental fluorosis should be seen as a warning sign for the development of skeletal fluorosis. However, no statistically significant relationships were found between dental fluorosis and BW, BMC or BMD in any of the 2 areas, which indicated that even life-long fluoride concentrations which are detrimental towards dental fluorosis (Table 1; mean fluorosis 3.6) had a relatively low influence on bone.
The insignificant differences in the BW (Fig. 1; Table 2) for gender and different fluoride areas indicated no influence of the 2 different water fluoride concentrations on growth. But the increase in bone width with age is normal during the growing phase of children.
The higher BMC in the 12-13 year age group in the high fluoride area can be explained by the fact that bone mass accrual accelerates more or less at these ages as a result of pubertal maturation. This would happen at 11 and 13 years of age, respectively for Western girls and boys [31] when bone mass increases at a high rate for three consecutive years in girls and four years in boys. Although our group can not be considered to fall exactly in the same Western category it provides an idea of growth effects and age.
Bone mineral density is now an acceptable measure for bone mass, the confirmation of osteoporosis and the risk of fragility fractures [15]. Bone mineral density (BMD) correlates well with the probability of fractures in people with osteoporosis as well as with the improvements in BMD following treatment for osteoporosis with a reduced rate of fracture [15]. In our study a higher BMD value was generally associated with more fluoride (3.00 mg/L) in the drinking water (Fig. 3). However, the bone density difference seems to become more evident at 12-13 years and even more so at 14-15 years of age. Furthermore, in general girls had higher density values than boys in the 12-13 year and 14-15 year age group of the high fluoride area (Fig. 3). Again this could be due to the earlier pubertal growth spurts in girls, with boys lagging behind until their growth spurt at or just before 14-15 years. However, it varied in the different age groups in the low fluoride area (Fig. 3). The bone density decreased significantly (p<0.02) from the 10 to 15 year age group in the 0.19 mg/L F area (Fig. 3). This might be due to the fact that as the children grow, the low amount of fluoride obtained from the drinking water consumed (F = 0.19 mg/L) was not enough to support an increase in bone density and the bone density started to decrease as a result of fluoride depletion. In contrast, the other higher fluoride (3 mg/L F) area might obtain relatively sufficient fluoride from water over the 10-15 year period. The bone density values reported in this study (Table 2) correspond in general to published values [14, 31]. From Fig. (3), it seems that after the age of 12 years (or after 12 years of fluoride intake) the difference in bone density becomes more evident as the graphs for the 2 different fluoride areas did not intersect.
In a study where the effect of long-term exposure to fluoride in drinking water (1.0 mg/L) on risks of bone fractures was investigated, a decrease in the risk of overall fractures was reported but not for hip fractures [32]. Furthermore, 33 Australian studies provide substantial evidence that fluoride up to 1 mg/L did not have an adverse effect on bone strength, bone mineral density or fracture incidence [15]. However, from the five cross-sectional studies it was concluded that usage of fluoridated water at 1 mg/L had a favorable effect on bone density [15], as was observed in our 3 mg/L fluoride area (Fig. 3). On the other hand, In another study where the drinking water had been fluoridated over a period of 30 years to 1 mg/L, no influence on bone density was found but a possibility in the reduction of osteoporotic hip fractures in the very old was reported [14]. A large body of epidemiological evidence exists in studies on different geographical areas and on different populations showing no adverse effect on bone from drinking fluoridated water (1 mg/L). Indeed the evidence would suggest it might be beneficial [8, 15]. Many studies indicated that fluoride in water at levels considered “optimal” for the prevention of dental caries ( around 1 mg/L F) increases bone mineral densities [10, 33-35] while others could not see an increase. In a long-term exposure [34], significantly higher bone density was reported in women for a 1.0 mg/L F area than for an area with 0.1 mg/L which indicated the effect of water fluoridation during the growing years. A high positive correlation was also found between the fluoride concentrations in water (0.32 mg/L, 1.69 mg/L and 2.74 mg/L) and bone density [33] as well as for women in Taiwan in areas with <0.6 and >1.0 mg/L fluoride [35]. In a study [36] on 15 and 17 years old, it was stated that the bone area explained most of the variance in BMC and BMD. In agreement with our study, it was also found [36] that the BMC was higher in the region with 10 times more fluoride (1.1 mg/L F) in the drinking water. A higher BMD was also reported [34] for long-term exposure to fluoridated water (1.0 mg/L F) compared to an area with 0.1 mg/L F for adulthood women. No differences between the two groups were found for height, weight, lifestyle or dietary factors.
As this study is a study on children in two very remote areas it can be expected to have some limitations. Unfortunately, there were no medical records available from where more information could have been extracted such as the incidence of bone fracture and bone fluorosis scores. Furthermore, the bone measurements could only be made at one point. In this study the Norland single energy photon absorptiometer was used as it is a portable apparatus and used by other researchers [25, 26]. However, it should be admitted that more sophisticated apparatuses are available but then they are mostly bulky and costly. However, even with this limitation significant trends were observed.
It can be anticipated that a dental fluorosis index (3.6; Table 1) found in the high fluoride area (3 mg/L) might be associated with the early-stage of skeletal fluorosis. This argument is based on a study [17] where a dental fluorosis index of 3.7 was associated with the early-stage of skeletal fluorosis and the report by the National Research Council [16] that a maximum drinking water fluoride standard of 4 mg/L is too high and does not protect against adverse health effects as skeletal fluorosis especially for life-long residents in these areas. Furthermore, this study did not show any relationship between fluorosis and bone mineral density at these 0.19 mg/L and 3.00 mg/L fluoride (which is still considered to be low in general medical terms) low drinking water fluoride levels. A strong positive linear relationship was detected between age and bone width of life-long resident children. The bone mineral density increased with age in a high fluoride area (3 mg/L) but decreased with age in the low fluoride area (0.19 mg/L).


