Effects of Aging and Cyclosporin A on Collagen Turnover in Human Gingiva
Abstract
Background:
We aimed at characterizing the aging gingiva analyzing: i) collagen content and turnover in human gingival tissues and fibroblasts obtained from healthy young and aging subjects. ii) the effect of cyclosporin A administration in human cultured gingival fibroblasts obtained from aging compared to young subjects.
Methods:
Morphological analysis was performed on haematoxylin-eosin and Sirius red stained paraffin-embedded gingival biopsies from young and aging healthy subjects. The expression of the main genes and proteins involved in collagen turnover were determined by real time PCR, dot blot and SDS-zymography on cultured young and aging gingival fibroblasts, and after cyclosporin A administration.
Results:
Our results suggest that in healthy aged people, gingival connective tissue is characterized by a similar collagen content and turnover. Collagen turnover pathways are similarly affected by cyclosporin A treatment in young and aging gingival fibroblasts.
Conclusions:
Cyclosporin A administration affects gingival collagen turnover pathways in young and aging fibroblasts at the same extent, suggesting that during aging cyclosporin A administration is not related to relevant collagen turnover modifications.
INTRODUCTION
The oral cavity is formed by different specialized tissues that synergistically concur at least in three essential physiological functions in humans: phonation, nutrition, and defence of the host against a variety of pathogens, i.e. xenobiotics, bacteria, viruses and mycetes. In healthy aging, these tasks are modestly affected, but oral health declines, possibly due to the age-related ability of the different tissues to maintain homeostasis. In fact, the oral cavity frequently manifests secondary effects of numerous systemic diseases and treatments during aging. The most relevant signs are a higher susceptibility to caries associated with xerostomia, periodontal inflammation and fungal infections. In this scenario, life style, medical conditions and therapeutic regimens, often concomitant in older persons, represent the major determinant. Moreover, poor oral health care, and dental or periodontal chronic infections could mediate weight change through alterations in diet and nutritional status, and also favour systemic effects [1-3].
Although a considerable amount of data are available regarding the effects of aging on the main tissues of the oral cavity, such as teeth, salivary glands, and periodontium [4, 5], very few studies investigated the age-related changes involving the gingiva [6].
A variety of age-related periodontal changes occur and, although controversial, earlier studies on aging gingiva showed that the metabolism of both collagen and non-collagenous proteins decreases with increasing age [7]. Age-dependent modifications were also found in the fibroblast size [8], and in the mitotic activity of both gingival fibroblasts and epithelial cells [9, 10].
Qualitative and quantitative changes in gingival connective tissue are prominent features of periodontal diseases. A disturbance in connective tissue homeostasis, mainly involving interstitial collagen as the major structural protein in gingival connective tissue, has been suggested as one of the most relevant events occurring in the development of drug-induced gingival overgrowth (GO), a pathology characterized by increased accumulation of interstitial collagen in the gingival connective compartment [11-13].
As previously reported, older people are susceptible to a higher incidence of multiple chronic disease and conditions compared with younger adults. As a consequence, they consume a greater number of prescription and non-prescription medications and, due to the age-associated changes of pharmacokinetic and pharmacodynamic properties, the elderly population is at greatest risk for medication-related problems that may very likely affect gingival connective homeostasis [14, 15].
Since the overall age-related gingival connective tissue modifications have still not been fully described [16], in the present study we combined different analytical approaches aimed at characterizing the complex mechanisms that control collagen turnover in aging gingival fibroblasts. In particular we aimed at: i) analyzing collagen content in young and aging gingivae, and the complex regulatory mechanisms that control collagen turnover in aging gingival fibroblasts using morphological and molecular approaches. ii) analyzing the effect of cyclosporin A on collagen turnover mechanisms in healthy aged gingival fibroblasts, compared to young ones in order to understand if older people are able to maintain tissue homeostasis or are more susceptible to the development of drug-induced gingival overgrowth.
This information might contribute to a better understanding of some of the modifications which gingiva undergoes with aging, providing a baseline to appraise the quantitative pathological changes of collagen turnover in disease states, and to understand the potential effect of pharmacologic treatment on gingival connective tissue.
MATERIALS AND METHODOLOGY
Experimental model
Gingival biopsies were obtained from 5 young (mean age 36 ± 9.23, 4 females and 1 male) and 7 aging subjects (mean age 70.17 ± 10.63, 4 females and 3 males). All subjects were no smokers, had clinically normal gingiva, with no signs of overt inflammation, hyperplasia and no history of drug use associated with GO, without any systemic health problems. Health, drugs, alcohol abuse and smoking histories were collected to exclude patients with known clinical situation able to affect the gingival status. They gave their informed consent to the biopsy, which was obtained from attached gingiva under local anaesthesia during minor oral surgical procedures. Each gingival biopsy was divided into two fragments: one fragment was processed for morphologic analysis and one fragment for cell culture evaluations.
Histochemistry and image analysis
Immediately after surgery, each gingival biopsy fragment was fixed in 10% formalin in 0.1M phosphate buffer saline (PBS), pH 7.4, for 5 hours at room temperature, routinely dehydrated, paraffin embedded, and serially sectioned (thickness 5 µm). Sections were stained with freshly made haematoxylin-eosin to evaluate the tissue morphology. To specifically stain collagen, slides were deparaffinized and immersed for 30 minutes in saturated aqueous picric acid containing 0.1% Sirius red F3BA (Sigma, Italy).
All of the Sirius red-stained sections were analyzed by light microscopy and the images were captured and digitized using an image analysis system with specific software (Bio Image Analyzer, ICH, Italy). This software automatically selects the collagenous portion on the basis of similarities in the colour of adjacent pixels, based on a RGB system. Tissue COL content is expressed by a fibrosis index (%), indicating the ratio of the mean Sirius red-stained surface to the whole area of the section.
Cell culture
Human gingival fibroblasts were obtained from the young and aging subjects. Gingival biopsies were washed with sterile PBS, plated in T-25 flasks, incubated in DMEM supplemented with 10% heat-inactivated fetal bovine serum (FBS) and antibiotics (100 U/mL penicillin, 0.1 mg/mL streptomycin) at 37°C in a humidified atmosphere containing 5% CO2. When fibroblasts grew out from the explant, they were trypsinized (0.25% trypsin-0.2% EDTA) for secondary cultures. Viability was assessed by the Trypan blue exclusion method. Confluent human gingival fibroblasts were used between the fourth and fifth passage.
Molecular evaluations were done on fibroblasts cultured for 72 h, using duplicate cultures for each sample.
Treatment of gingival fibroblasts with CsA:
When human gingival fibroblasts grew to confluence in T-75 flasks, the culture medium was replaced with serum-free DMEM containing 2 doses of CsA (252 and 800 ng/ml) dissolved in a vehicle (0.008% ethanol, 0.0016% Tween-20). The lower dose corresponds to the whole blood trough levels of CsA. The higher dose was reported to induce modifications of proliferation and ECM turnover, and to affect collagen homeostasis in gingival fibroblasts obtained from young healthy subjects [12]. The cultures were then incubated at 37°C for 72 h. To assess CsA effect, cultured fibroblasts of each sample incubated in CsA vehicle served as controls (VH). At the established intervals of time the cell culture supernatants were collected and fibroblasts were washed in PBS, trypsinized and harvested by centrifugation (100 xg, 5 min).
Real-time RT-PCR
Total RNA was isolated by a modification of the acid guanidinium thiocyanate-phenol-chloroform method (Tri-Reagent, Sigma, Italy). One µg of total RNA was reverse-transcribed in 20 µL final volume of reaction mix (Biorad, Italy). mRNA levels of collagens type I (COL-I), long lysyl hydroxylase 2 (LH2b), matrix metalloproteinase 1 (MMP-1), tissue inhibitor of MMP (TIMP-1), Secreted Protein Acidic and Rich in Cysteine (SPARC), transforming growth factor-β1 (TGF-β1) were assessed.
The primers sequences, designed with Beacon Designer 6.0 Software (BioRad, Italy), were the following: GAPDH: sense CCCTTCATTGACCTCAACTACATG, antisense TG GGATTTCCATTGATGACAAGC; COL-I: sense CGACCTGGTGAGAGAGGAGTTG, anti-sense AATCCATCCAGACCATTGTGTCC; MMP-1: sense CGGATACCCCA AGGACATCTACAG, antisense GCCAATTCCAGGAAA GTCATGTGC; TGF-β1: sense GTGCGGCAGTGGTTG AGC, antisense GGTAGTGAACCCGTTGATGTCC; TIMP-1: sense GGCTTCTGGCATCCTGTTGTTG, antisense AAGGTGGTCTGGTTGACTTCTGG; LH2b: sense CCG GAAACATTCCAAATGCTCAG, antisense GCCAGAGGT CATTGTTATAATGGG; SPARC: sense GCGAGCTG GATGAGAACAACAC, antisense GTGGCAAAGAAGT GGCAGGAAG.
GAPDH was used as endogenous control to normalize the differences in the amount of total RNA in each sample. Amplification reactions were conducted in a 96-well plate in a final volume of 20 µL per well containing 10µL of 1x SYBR Green Supermix (BioRad, Italy), 2 µL of template, 300 pmol of each primer, and each sample was analysed in triplicate. The cycle threshold (Ct) was determined and gene expression levels relative to that of GAPDH were calculated by the 2-ΔΔCt method, according to Livak and Schmittgen [17].
Slot blot
Cell culture media from young and aging fibroblasts were concentrated 20-fold with Centricon 10 columns (Amicon Y10, Millipore, Italy). Protein content was determined by a standardized colorimetric assay (DC Protein Assay, Bio Rad, Italy); 20 µg of total protein per sample in a final volume of 200 µL of Tris buffer saline (TBS) were spotted onto a nitrocellulose membrane in a Bio-Dot SF apparatus (Bio-Rad, Italy). Membranes were blocked for 1 h with 5% skimmed milk in TBST (TBS containing 0.05% tween-20), pH 8, and incubated for 1 h at room temperature in monoclonal antibody to COL-I (1:1000 in TBST) (Sigma, Italy) or to COL-III (1:2000 in TBST) (Sigma, Italy). After washing, membranes were incubated in HRP-conjugated rabbit anti-mouse serum (1:80,000 in TBST) (Sigma, Italy) for 1 h. Immunoreactive bands revealed by the Amplified Opti-4CN substrate (Amplified Opti-4CN, Bio Rad, Italy) were scanned densitometrically (UVBand, Eppendorf, Italy).
MMP-1 protein expression in VH and CsA treated fibroblasts was assessed by slot blot. The membranes were incubated overnight at 4°C in monoclonal antibody to MMP-1 (1 µg/ml in TBST, Calbiochem) and, after washing, in HRP-conjugated rabbit anti-mouse serum (1:80,000 dilution, Sigma).
SDS-zymography
Concentrated culture media were mixed 3:1 with sample buffer (containing 10% SDS). Samples (five µg of total protein per sample) were run under non-reducing conditions without heat denaturation onto 10% polyacrylamide gel (SDS-PAGE) co-polymerized with 1 mg/mL of type I gelatin. The gels were run at 4°C. After SDS-PAGE, the gels were washed twice in 2.5% Triton X-100 for 30 min each and incubated overnight in a substrate buffer at 37°C (Tris-HCl 50 mM, CaCl2 5 mM, NaN3 0.02%, pH 7.5). The matrix metalloproteinase (MMP) gelatinolytic activity was detected after staining the gels with Coomassie brilliant blue R250, as clear bands on a blue background.
Western blot
Concentrated culture media (5 µg of total proteins) were diluted in SDS-sample buffer, loaded on 10% SDS-polyacrylamide gel, separated under reducing and denaturing conditions at 80 V according to Laemmli, and transferred at 90 V to a nitrocellulose membrane in 0.025 M Tris, 192 mM glycine, 20% methanol, pH 8.3. After electroblotting, the membranes were air dried and blocked for 1 hour. After being washed in TBST (TBS/Tween-20 0.05%), membranes were incubated for 1 h at room temperature in monoclonal antibody to SPARC (1:100 in TBST, Novocastra Laboratories) and, after washing, in HRP-conjugated rabbit anti-mouse serum (1:6000 dilution, Sigma, Italy). Immunoreactive bands were revealed using the Opti-4CN substrate Bio Rad.
Statistical analysis
All amplifications were run in triplicate, and all of the evaluations were repeated two times. To compare the young and aging untreated fibroblasts, data were analyzed by an unpaired t-test, and the age was the independent variable. The hypothesis was 2-tailed. To assess the effects of aging and CsA administration, data were analyzed by 2-way ANOVA. p values less than 0.05 were considered significant. A post hoc assessment of the power of the t-test [18] and the variance test made using the non-central F-distribution [19] was performed.
RESULTS
Our first aim was to characterize collagen content and turnover in both gingival tissues and cultured gingival fibroblasts obtained from healthy young and aging subjects.
Morphological and quantitative image analysis:
Under light microscopy haematoxylin-eosin stained sections of young and aging samples appeared similar (Fig. 1a, b). In the epithelium of the two experimental groups we observed a similar number of the living cell layers, excluding the presence of acanthosis. The rete peg was similarly prolonged in both experimental groups, and regularly elongated. The connective tissue included a similar amount of collagen fiber bundles running in all directions, with some inflammatory cell infiltration which tended to be more abundant in some of the aging gingival fragments.
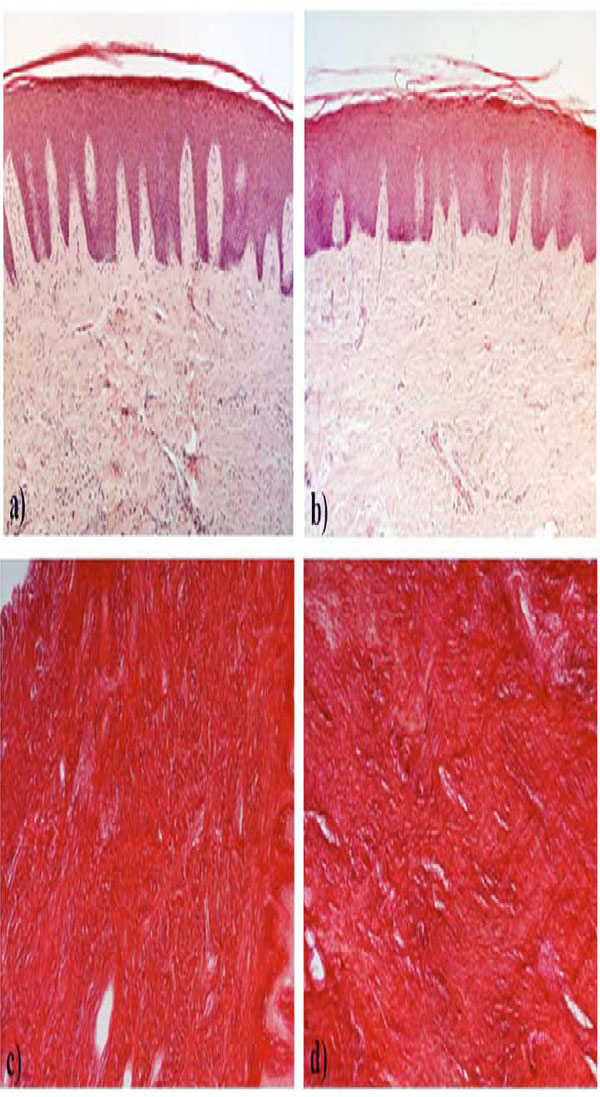
Microphotographs of young and aging gingiva paraffin-embedded sections stained with hematoxylin-eosin (a, b) or with Sirius red (c, d). a and c: young gingiva; b and d: aging gingiva. Original magnification: 10X.
Sirius red stained the collagen specifically and computerized analysis of sections of gingiva from young and aging subjects indicated that the fibrosis index was, respectively, 83.5% ± 6.29 and 82.5% ± 4.74 (Fig. 1c, d).
Gene expression
mRNA levels are shown in Fig. (2a). COL-I gene expression decreased in aging fibroblasts compared to the young ones (p<0.05). A tendency to decrease was observed for TIMP-1 (p = 0.068), while MMP-1 and TGF-β1 mRNA levels were unchanged (p ns). LH2b mRNA levels in aging fibroblasts were unaffected. Interestingly, if we consider the LH2b/COL-I mRNA ratio, there was a significant increase (p< 0.05) of this ratio in aging fibroblasts compared to young ones.
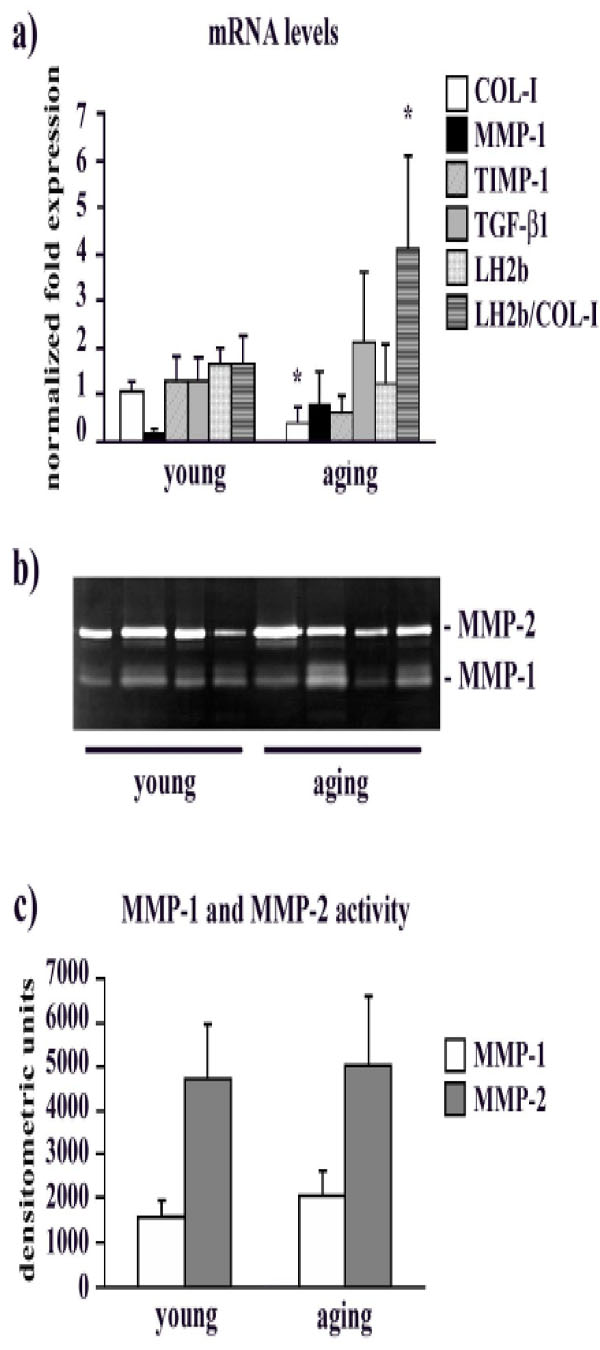
a) Bar graphs showing COL-I, MMP-1, TIMP-1, TGF-β1 and LH2b mRNA levels, and the LH2b/COL-I mRNA ratio in young and aging cultured fibroblasts. Changes in mRNA are normalized on GAPDH gene expression. Values are means ± SD for duplicate samples. * indicates a significant difference (p < 0.05, unpaired Student’s t test). b) Representative gelatin zymogram of MMPs in serum-free, conditioned human gingival fibroblast supernatants from young and aging gingival. The lytic bands weighing 66 kDa and in the 60/50 kDa region correspond to proMMP-1 and proMMP-2, respectively. c) Bar graphs showing proMMP-1 and proMMP-2 levels in fibroblast serum-free conditioned media after densitometric analysis of lytic bands following SDS-zymography. Data are expressed as densitometric units and are means ± SD.
COL protein levels
COL-I and COL-III protein levels were assessed in fibroblast supernatants by slot blot. Densitometric scanning of immunoreactive bands showed that COL-I and COL-III content was not affected by aging (data not shown).
COL degradation
MMP-1 and MMP-2 protein levels in young and aging fibroblast supernatants is presented in Fig. (2b, 2c). The densitometric analysis of lytic bands identified as MMP-1 revealed similar levels of interstitial collagenase in the supernatants obtained from young and aging subjects (Fig. 2b, c). This result was confirmed by slot blot analysis (data not shown). Also MMP-2 protein levels seems unaffected by aging (Fig. 2b, c).
SPARC expression
SPARC mRNA levels tended to be lower in aging compared to young fibroblasts (0.46 ± 0.08 and 0.96 ± 0.079, respectively, p ns). SPARC protein levels, however, were not significantly affected by the aging process (young 195095 ± 110644, aging 170894 ± 173511, p ns).
Post hoc power assessment of the statistical test revealed that the power was >0.90 for COL-I and SPARC gene expression, indicating that the analysis had not type II errors. The power was comprised between 0.4 and 0.6 for MMP-1 mRNA, TIMP-1 mRNA and LH2b/COL-I mRNA ratio, and was <0.2 for LH2b and TGF-(β1 mRNA levels, indicating that the analyses had a type II errors.
Effect of CsA on Collagen Turnover in Young and Aging Fibroblasts
Our second aim was to characterize if collagen turnover was affected by CsA administration at a different extent in cultured gingival fibroblasts obtained from healthy young and aging subjects.
Gene expression
COL-I gene expression (Fig. 3a) was not affected by CsA administration both in young and in aging fibroblasts (p [age] < 0.0005, p [treatment] = ns, p [interaction]= ns). A similar pattern was observed for MMP-1 (p [age] = ns, p [treatment] = ns, p [interaction]= ns) (Fig. 3b), TIMP-1 (p [age] < 0.05, p [treatment] = ns, p [interaction]= ns) (Fig. 3c), TGF-β1 (p [age] = ns, p [treatment] = ns, p [interaction]= ns) (Fig. 3d), LH2b (p [age] = ns, p [treatment] = ns, p [interaction]= ns) (Fig. 3e), and SPARC mRNA levels (p [age] = ns, p [treatment] = ns, p [interaction]= ns) (Fig. 3f).
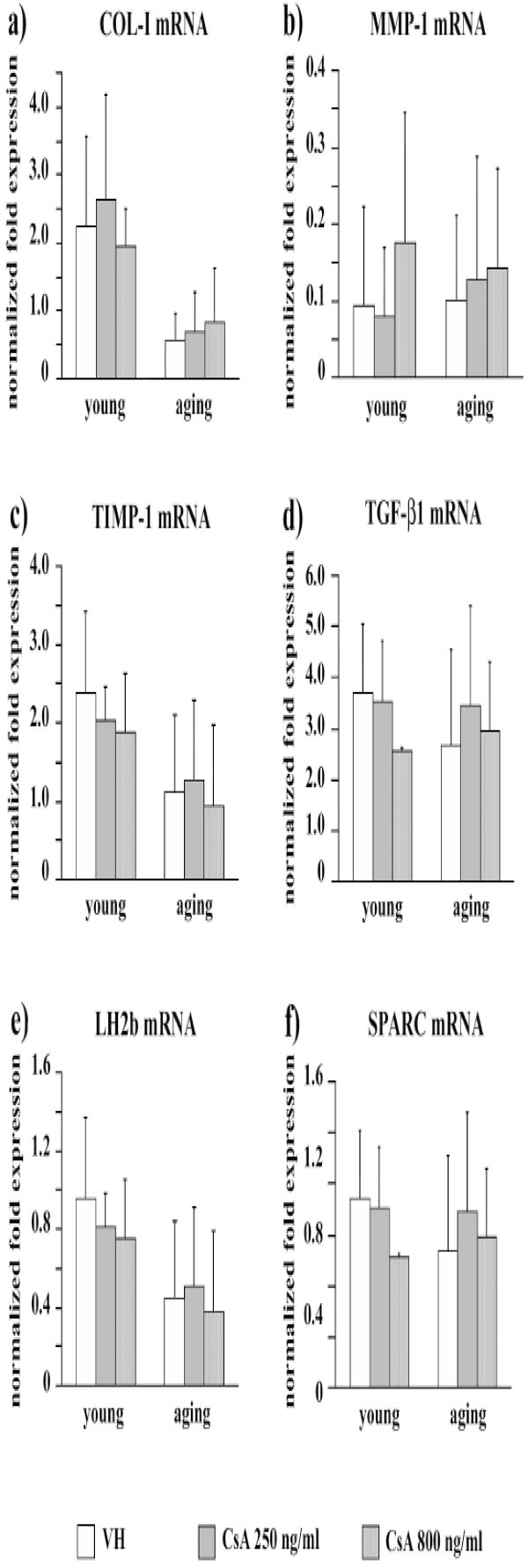
Bar graphs showing mRNA levels for COL-I (a), MMP-1 (b), TIMP-1 (c), TGF-β1 (d), LH2b (e), and SPARC (f) in VH and CsA-treated gingival fibroblasts obtained from young and aging healthy donors. mRNA are normalized on GAPDH gene expression. Values are means ± SD for duplicate samples. VH: vehicle.
COL protein levels
COL-I protein levels were assessed in fibroblast supernatants by slot blot. Densitometric scanning of immunoreactive bands showed that COL-I levels tended to be higher in CsA-treated young fibroblasts, and were not modified by CsA in aging fibroblasts (Fig. 4a).
COL degradation
MMP-1 protein levels in young and aging fibroblast supernatants is presented in Fig. 4b. MMP-1 protein levels tended to be down-regulated in CsA-treated young fibroblasts, compared to VH. In aging fibroblasts we observed a wide interindividual variation and a tendency to decrease after 800 ng/ml CsA, compared to VH (p [age] = ns, p [treatment] = ns, p [interaction]= ns). MMP-2 activity was unchanged after CsA administration in both young and aging fibroblasts (p [age] = ns, p [treatment] = ns, p [interaction]= ns) (data not shown).

Bar graph presenting COL-I (a), MMP-1 (b), and SPARC (c) protein levels VH and CsA-treated gingival fibroblasts obtained from young and aging healthy donors, assessed by slot blot or Western blot analysis, as described in the Material and Methods section. Data are reported as densitometric units after scanning of the immunoreactive bands. Values are means ± SD for duplicate samples. VH: vehicle.
SPARC expression
SPARC protein levels were unchanged by CsA administration in both young and aging gingival fibroblasts (p [age] = ns, p [treatment] = ns, p [interaction]= ns) (Fig. 4c), as observed for SPARC mRNA levels (p [age] = ns, p [treatment] = ns, p [interaction]= ns).
Post hoc power assessment of the statistical test revealed that the power was comprised between 0.88 and 0.99 for COL-I mRNA and protein levels, MMP-1 mRNA and protein levels, and TIMP-1 mRNA levels, suggesting that the analysis had not type II errors. For the remnant evaluations, the power was comprised between 0.30 and 0.40, indicating that the analyses had type II errors.
DISCUSSION
Chronic diseases are common among older people and increase the necessity for multiple medications. Polipharmacotherapy, together with the well established changes of the pharmacokinetic processes, render the elderly more susceptible to the adverse effects of drugs [20].
Periodontal tissue is susceptible to a range of systemic medications able to promote unwanted effects (e.g. GO), and the gingiva may be therefore a target of adverse reactions, particularly in the elderly.
Collagen is the main component of gingival connective compartment. Its turnover is a vital step in the tissue remodelling that accompanies physiological and pathological processes, and its age-dependent imbalance is a hallmark of the aging of various organs, i.e. heart and the kidney [21, 22], but very few data are available regarding the gingiva.
Gingival connective tissue undergoes a rapid rate of collagen turnover [23], therefore all of the mechanisms involved (collagen synthesis, maturation, and degradation) are of considerable importance in the net collagen steady-state, in which MMPs play a pivotal role [24]. MMPs with collagenase and gelatinase activities are secreted in the extracellular space as zymogens and are activated by proteolytic cleavage; their activity is closely regulated and under pathological conditions any mismatch could result in excessive ECM accumulation or degradation.
Interstitial collagenase or MMP-1 cleaves the native triple helical region of interstitial COL into characteristic 3/4- and 1/4-collagen degradation fragments [25], allowing its further digestion by less specific proteinases, leading to complete digestion of fibrillary COL.
The extracellular activity of MMPs is tightly regulated at various points, including inhibition by TIMPs, among which TIMP-1 and 2 are the most important since they inhibit the active form of all MMPs, forming inactive 1:1 stoichiometric TIMP-1/MMP-1 complexes [26, 27].
During COL maturation, post-translational hydroxylation of COL lysine residues, accomplished by lysyl hydroxylase (LH) is a key mechanism influencing collagen biosynthesis and extracellular matrix stability [28]. Two alternately-spliced forms of LH2 exist, the long (LH2b) and the short (LH2a) ones [28]. We investigated LH2b gene expression, since it is the major form expressed in all tissues and is generally overexpressed in fibrotic processes [29]. LH2b, in fact, is responsible for the over-hydroxylation of the COL telopeptides, forming COL pyridinoline cross-links, thus contributing to unwanted COL accumulation [29].
Our first aim was to characterize collagen turnover pathways in untreated aging gingival fibroblasts, compared to young ones. COL content, as shown by the fibrosis index and protein analysis results, is similar in young and aging subjects. Moreover, our results show unchanged MMP-1 as well as TIMP-1 mRNA levels, and similar MMP-1 and MMP-2 protein levels, suggesting that gingival collagen content is not regulated at the level of its degradation. This was consistent by the increased LH2b/COL-I mRNA ratio (indicating the gene expression of LH2b relative to COL-I). Although the LH2b/COL-I mRNA ratio increased due to decreased COL-I mRNA levels with age, we can hypothesize that the newly synthesized collagen could be more susceptible to be cross-linked in aged compared to young gingival fibroblasts, and therefore less susceptible to degradation [30]. This hypothesis is consistent with the finding of COL-I mRNA levels down-regulation in aging fibroblasts, of a similar fibrosis index and interstitial collagen content in both the experimental groups, together with a similar MMPs activity, suggesting that in young and aging gingival collagen regulation may be very likely dependent in part on the maturation pathways carried out by post-translational modifications such as collagen crosslinking.
The overall balance of COL turnover is controlled also by TGF-β1, a multifunctional cytokine known to be involved in both healing and fibrogenic processes, being capable of regulating cell proliferation and differentiation as well as directly activating gene expression for the synthesis of ECM components [31]. Also TGF-β1 gene expression was unchanged in aging fibroblasts, suggesting a similar tone of TGF-β1 influencing collagen turnover.
SPARC is an important multifunctional glycoprotein that modulates cellular interactions with ECM by its binding to structural matrix proteins [32]. In adults, the expression of SPARC is limited to tissues undergoing repair or remodelling, and healthy gingival connective tissue is a remodelling tissue with high turnover [33, 34]. Since SPARC protein levels seem not significantly affected by the aging process, we can hypothesize that gingival connective tissue remodelling occurs at a similar extent in young and aged people.
Our second aim was to analyze the effect of CsA on collagen turnover in healthy aging gingival fibroblasts, compared to young ones. Our results show that collagen turnover pathways are similarly affected by CsA treatment in young and aging gingival fibroblasts. Aging induced the increase of collagen protein levels and TIMP-1 mRNA down-regulation, but we did not find any statistical difference due to aging-CsA interaction, and therefore the described differences between young and aging CsA-treated fibroblasts are due to aging itself and not to CsA administration. Considered as a whole, these findings suggest that young and aging gingival fibroblasts respond to CsA administration at the same extent.
CONCLUSION
Considered as a whole, the present study offers new insights on the relationship between aging and gingival connective tissue homeostasis. Our results show that in the absence of any critical disorder in healthy aged people, gingival connective tissue is characterized by a similar collagen content and turnover, suggesting that in the senescent phenotype, the complex machinery that controls collagen turnover is balanced. We hypothesized that, since in the elderly the defence machinery against the xenobiotic is weakened, and since the elderly use a higher number of medications, they are more susceptible to drug effects due to diminished ability to maintain tissue homeostasis. In particular, interstitial collagen is the main component of gingival connective tissue and its turnover is the target of several drugs leading to GO, such as CsA. Although collagen turnover is the main target of CsA in gingival fibroblasts, our results show that during aging, CsA administration is not related to relevant collagen turnover modifications, and that CsA treatment affects collagen turnover pathways at a similar extent in young and aging gingival fibroblasts.
The post hoc assessment of the power of the study revealed that in some occasions a type II error was committed. As a consequence, this study presents a limit due to the number of the analyzed subjects: the lack of statistical significance should be taken with caution. However, our results concerning the effect of CsA administration on young and aging healthy gingival fibroblasts are consistent since COL-I and MMP-1 are the main targets of CsA, and the power of the study for these two parameters was > 0.88.
ACKNOWLEDGEMENTS
We would like to thank Dr. Marco Giorgio Bianchi (Bio-Rad Laboratories System Specialists – Gene eXpr. Division) for excellent technical assistance, and the Ariel Foundation for financial support.


