All published articles of this journal are available on ScienceDirect.
Structural and Ultra-Structural Features of the First Mandibular Molars of Young Rats Submitted to Pre and Postnatal Protein Deficiencies
Abstract
The effects of protein malnutrition, both in utero and prior to weaning, on formation of the first mandibular molars were evaluated by phase-contrast and electron microscopy in rats. The nourished group (GI) received a diet that included 20% casein, while the malnourished group (GII) received 5% casein. The first mandibular molars from GII exhibited low density of cells and odontoblasts, which lacked regular organization compared with molars from GI. In addition, a difference in collagen type was observed between the groups, with a prevalence of Type III collagen fibers detected in the dentin, periodontal ligament, and alveolar bone of GII, and a prevalence of Type I collagen fibers in GI. Finally, examination of surface area in molar sagittal sections indicated 30% less dentin in GII, compared with GI. Our results suggest that structural and ultra-structural features of the dentin-pulp complex and periodontal components of rat molars are affected by protein deficiency.
INTRODUCTION
Nutritional protein deficiency can affect multiple tissues, organs, and systems [1-4]. In mineralized structures, such as bone, protein deficiency can lead to a delay in the appearance of ossification centers as well as a decrease bone width [5-8]. Nonetheless, the length of long bones, which exhibit extended growth, are less affected by nutritional insult [3].
For teeth, disturbances in diet during enamel and dentin formation lead to changes in the chemical composition, cellular structure, and consequently appearance, of the teeth [9], and the relationship between tooth size and morphology and tooth development and maturation has been previously described [10]. Importantly, malnutrition can lead to a delay in dental eruption [11-15] and an increase in susceptibility to development of caries [11,16-18]. Dental germs of rats submitted to protein deficiency exhibited weight reduction, lower calcium levels [19], and a substantial decrease in collagen synthesis [20]. Fluoride deficiency in rats maintained on a low-protein diet increased enamel deposition by 48% and decreased dentin by 28% [21]. Moreover, reduced dentin formation was observed among rats submitted to a protein-deficient diet [22]. Recently, the effects of undernutrition on enamel defects in socio-economically underprivileged communities have begun to be appreciated [23].
Given these descriptive studies of the effects of malnutrition on dental structure, we here aimed to evaluate structural and ultra-structural features of mineralized dental tissues, the dentin-pulp complex, and periodontal structures, as well as the sectional area of dentin in molars from rats submitted to pre and postnatal protein deficiencies.
MATERIALS AND METHODOLOGY
Experiments were conducted according to Ethical Principles in Animal Research adopted by the Brazilian College of Animal Experimentation (COBEA). Young male and female Wistar rats (200-240 g body weight) were mated for seven to ten days. After conception, females were placed in individual cages and were maintained under standard conditions at 21°C, with a 12 h light–dark cycle. During gestation, rats were given ad lib access to water and to AIN-93G purified rodent diet [24]. The diets for the control or nourished (GI) and malnourished (GII) groups contained 20% and 5% content of casein, respectively (Rhoster, SP, Brazil). Following birth, dams and pups of both groups, received respective diets until weaning (21 days).
Five male pups from both GI and GII groups were killed by intraperitoneal injection of 3% hypnol. The soft tissues surrounding the mandibles were dissected, and were sectioned along the midline. Right and left hemi-mandibles from each specimen were immersed for fixation in Bouin solution for 48 hours, washed in running water for 18 hours, and maintained in fixative solution of (50% formic acid and 20% sodium citrate) [25]. After dehydration through an increasing alcohol series, specimens were embedded in paraplast (Histologie Histosec – Merck). Sagittal serial sections of 6 µm were obtained from hemi-mandibles, and were stained with Hematoxilin-eosin by Masson’s technique. Samples were also using the Picro-sirius method [26], and were examined under polarized light.
For scanning electron microscopy (SEM), mandibles from five male pups from each group were obtained, dissected as previously described, and immersed in a modified solution of Karnovsky solution (2.5% glutaraldehyde and 2% paraformaldehyde in 0.1 M sodium phosphate buffer, pH 7.3) for 24 hours at 4ºC. After sectioning along the midline, right and left hemi-mandibles from each specimen were immersed in a 0.5% sodium hypochlorite solution for 3 days, washed in distilled water, and dehydrated through an increasing alcohol series. Specimens were then dried using a CO2 critical point device, mounted on aluminum stubs with conductive glue, gold sputtered, and then examined by SEM (Jeol, JSM-6100). The right first lower molar from three animals from each group were prepared for transmission electronic microscope (TEM). These were immersed in a fixative solution (2.5% glutaraldehyde and 0.2% tannic acid). After centrifugation for 30 min, specimens were again fixed in glutaraldehyde/tannic acid for 2 hours, and then immersed in a decalcifying solution (50% formic acid and 20% sodium citrate). Specimens were embedded in araldite using standard procedures and examined by TEM (Phillips EM-301 electron-microscope).
Morphometric analysis of hemi-mandibles from both groups was performed using hematoxylin-eosin staining. The Axiovision system (Zeiss) was used to make internal and external dentin surface drawn. Mean dentin surface areas in sagittal sections (in mm2) for the first lower molars from both groups was determined using a morphometric method [27]. Data were analyzed by Student’s t-test (significance = p < 0.05).
RESULTS
Phase-Contrast Microscopic Examination of Molars
We examined dentin in the first molars from animals submitted to normal and low protein diets. In animals from GI, the control group, a thick odontoblastic palisade was observed lining the dentin in the radicular region of the first molars. Near the pulpar cavity, a narrow cell-free and a well-defined inner zones rich in fibroblasts and mesenchymal cells were also observed (Fig. 1A). In contrast, in GII first molars, the odontoblastic palisade was not observed, nor could a cell-free zone be detected (Fig. 1B).
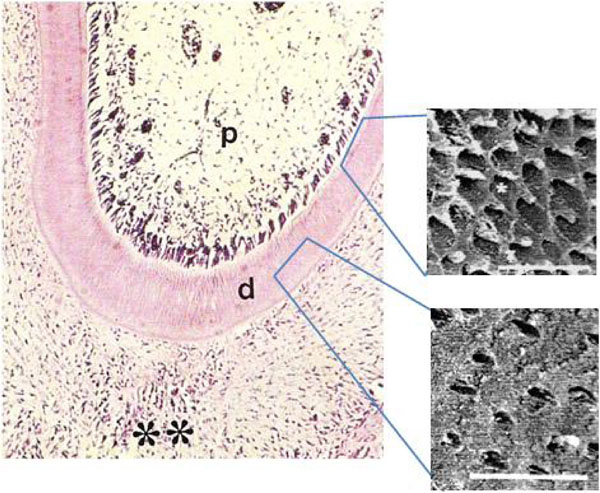
Photomicrograph of rat molars. Radicular region of GI molar showing the dental pulp (p), dentin (d) and periodontum (**). Odontoblastic palisade lining the dentin (Calibration bar: 50 µm). SEM reveals three-dimensional features including circumpulpal and peripheral dentin with dentinal tubules (*). (Calibration bar: 10 µm).
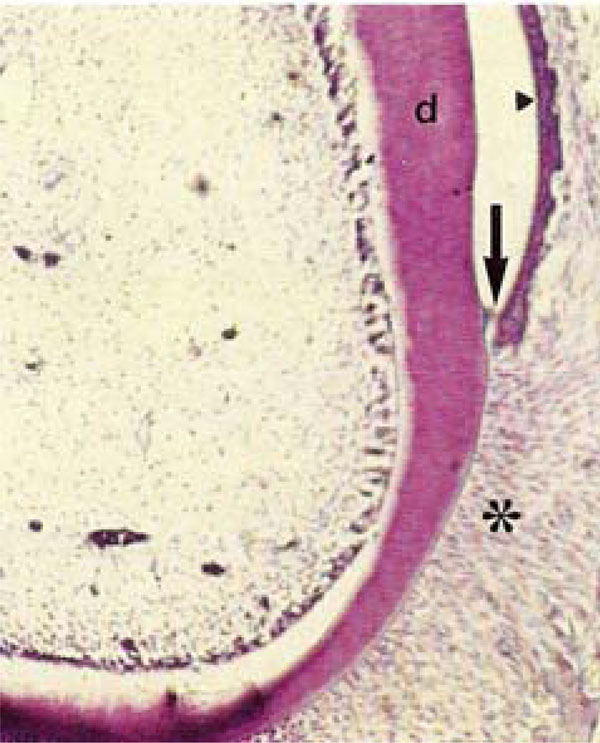
Photomicrograph of rat molars. In GII molars, no odontoblastic palisade or cell-free zone was observed. Note in the gingival sulcus, the sulcular epithelium (arrowhead) and loss of epithelial adhesion (arrow) within the junctional epithelium (HE, Calibration bar: 50 µm).
Pulp tissue, which is well organized and highly cellularized in molars from GI, exhibited broad hollow spaces in the first molars of GII animals (Fig. 2A,B). At high magnification, the more densely organized cellular components in the pulp of nourished animals (GI) was apparent (Fig. 2C, D).
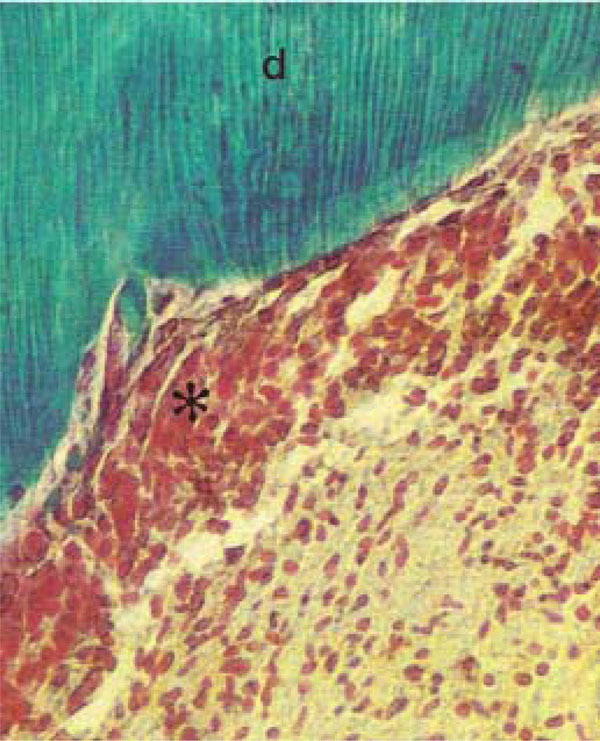
Photomicrograph of rat molars. In GI molars, dentin (d) with parallel dentinal tubules are detected, and dental pulp (*) cellular elements appear highly organized (Tricromo Masson, Calibration bar: 50 µm).
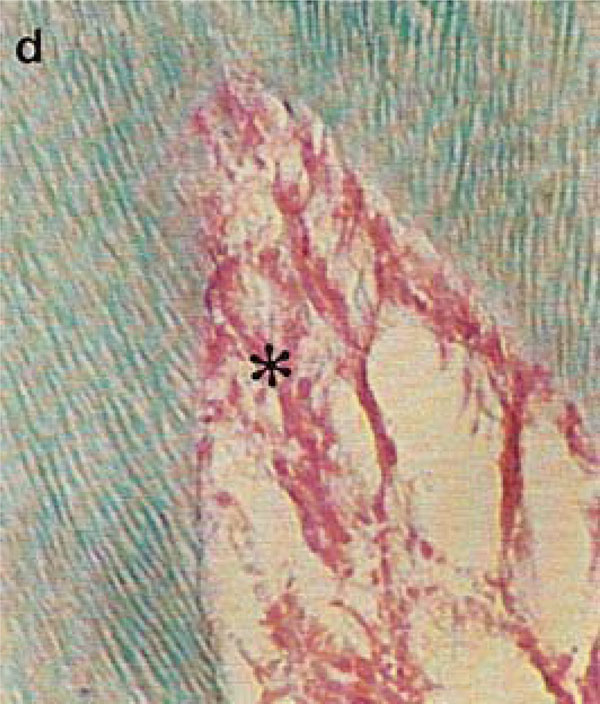
Photomicrograph of rat molars. In GII molars, parallel dentinal tubules (d) are accompanied by retracted pulp (*) with wide interior spaces (Tricromo Masson, Calibration bar: 50 µm).

Photomicrograph of rat molars. In GI molars, fibroblasts are detected within the central pulp, and exhibit granular cytoplasm and an eccentric nucleus (arrows) (HE, Calibration bar: 20 µm).
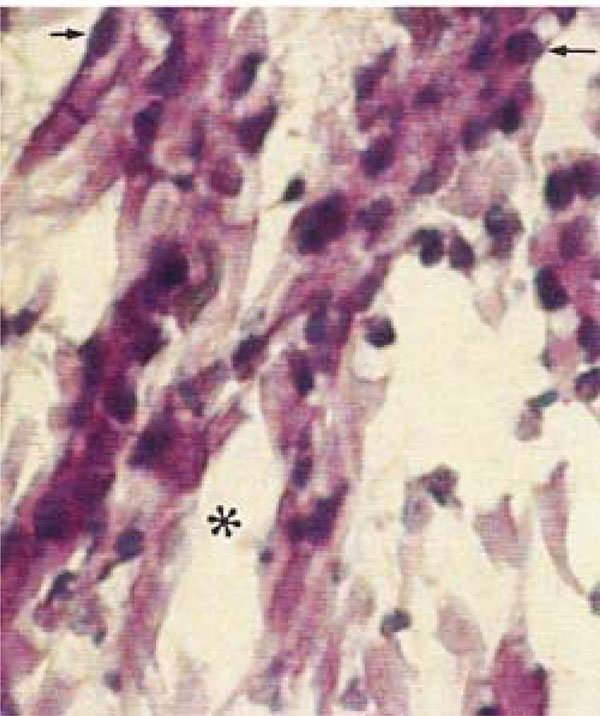
Photomicrograph of rat molars. In GII molars, broad spaces are detected within the central pulp (*), and fibroblasts exhibit irregular morphology, with retracted and intensely stained nuclei (arrow) (HE, Calibration bar: 20 µm).
In the gingival sulcus of molars of GI, the junctional epithelium exhibited well-defined cell outlines that were not be observed in GII molars. In contrast, a loss of epithelial adherence was evident in GII molars (Fig. 1B, 3A, B). Densely arranged fibroblasts were present in the periodontal ligament of mandibles from GI. These cells exhibited elongated, parallel nuclei that were oriented in the same direction as the collagen fibers within the ligament (Fig. 3A, C). In GII, fibers lacked regular organization, and the contours of the fibroblasts nuclei were not well delineated (Fig. 3D).
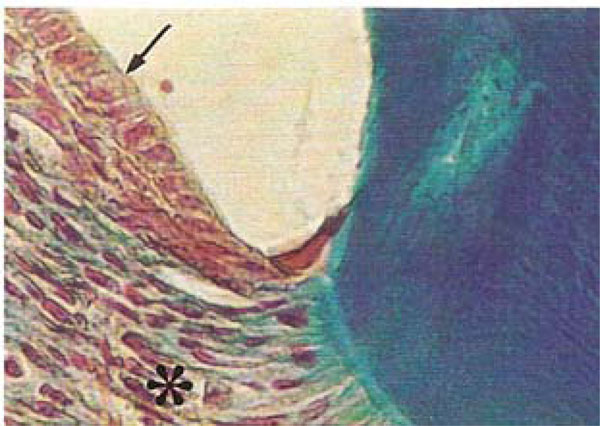
Photomicrograph of the periodontal region of rat molars. Within GI molars, dense areas of the periodontal ligament (*) appeared rich in fibroblasts and distinct layers were evident within the junctional epithelium (arrow) (Tricromo Masson, Calibration bar: 50 µm).

Photomicrograph of the periodontal region of rat molars. Within GII molars, the periodontal ligament (*) stained weakly, and few fibroblasts were detected. In addition, few cellular layers were detected within the junctional epithelium (arrow) (Tricromo Masson, Calibration bar: 50 µm).

Photomicrograph of the periodontal region of rat molars. Within GI molars, fibroblasts within the periodontal ligament (larger arrows) are arranged in parallel in the direction of the collagen fibers (larger arrows) (Tricromo Masson, Calibration bar: 10 µm).
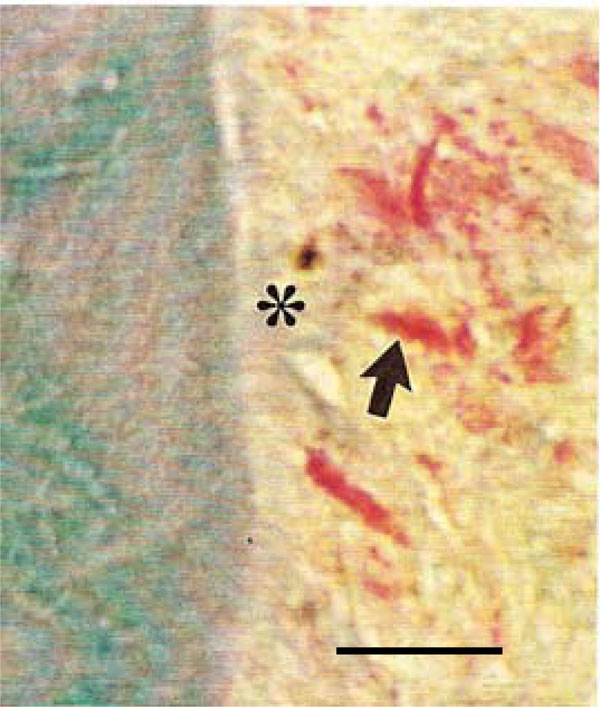
Photomicrograph of the periodontal region of rat molars. Within GII, collagen fibers appear disorganized (*), and weakly stained fibroblasts with indistinct nuclei (arrow) were detected (Tricromo Masson, Calibration bar: 10 µm).
Analysis of sections stained with the Picro-sirius method under polarized light allowed observation of dentin, periodontal ligaments, and alveolar bone regions in both groups. Within GI Type I collagen fibers were almost exclusively detected (red, orange and yellow). In the corresponding regions of GII, in spite of the presence of Type I collagen fibers in dentin, Type III collagen fibers were clearly predominant (green). In addition, the trabecular structure of the alveolar bone in GI was thicker than in GII, with a predominance of Type I collagen fibers detected in GI (Fig. 4E, F).
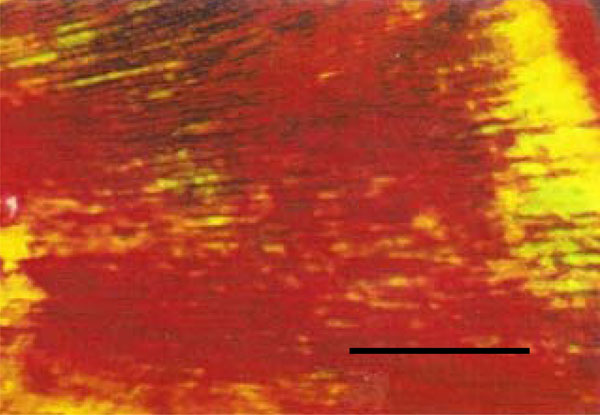
Photomicrograph of rat molar under polarized light. Within GI molars, mainly Type I collagen fibers are detected in the dentin (Picro-sirius, Calibration bar: 50 µm).
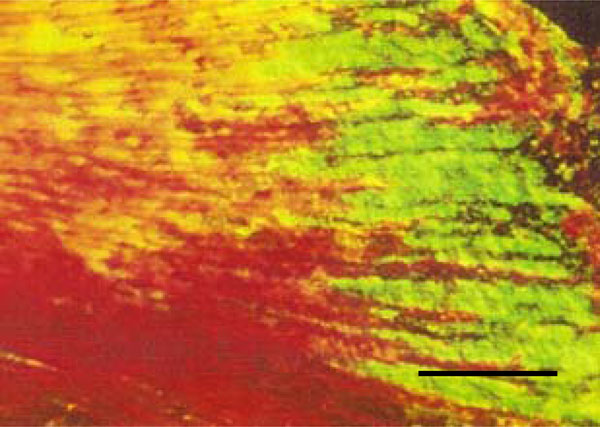
Photomicrograph of rat molar under polarized light. Within GII molars, Types I and III collagen fibers are detected in the dentin (Picro-sirius, Calibration bar: 50 µm).
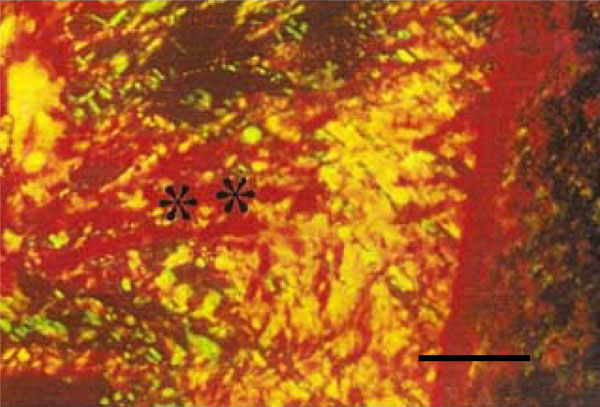
Photomicrograph of rat molar under polarized light. Within GI periodontal ligaments, mainly type I are detected (**) (Picro-sirius, Calibration bar: 50 µm).
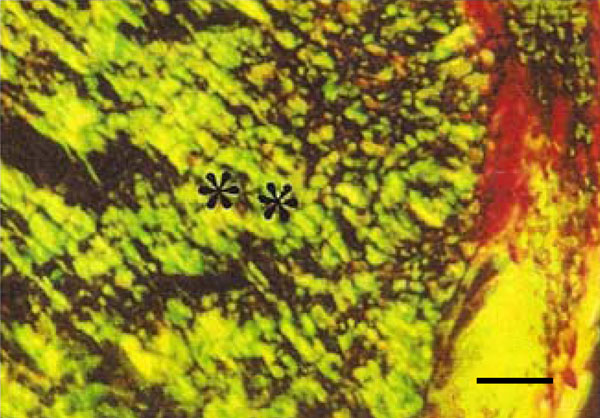
Photomicrograph of rat molar under polarized light. Within GII periodontal ligaments, mainly Type III collagen fibers are detected (**) (Picro-sirius, Calibration bar: 50 µm).
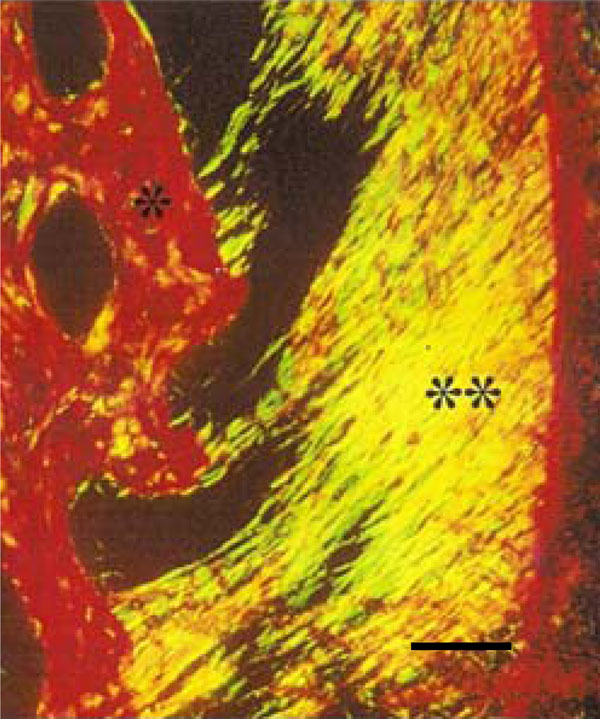
Photomicrograph of rat molar under polarized light. Within GI, predominantly Type I collagen fibers are detected in the periodontal ligament (**) and alveolar bone (*) (Picro-sirius, Calibration bar: 50 µm).
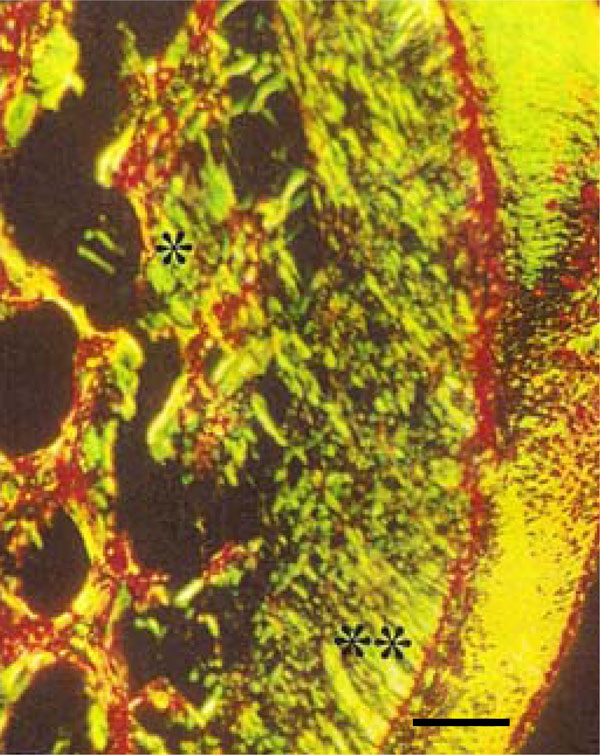
Photomicrograph of rat molar under polarized light. Within Photomicrography of rat molar under polarized light. GII, Type III collagen fibers are mainly detected in periodontal ligament (**) and alveolar bone (*) (Picro-sirius, Calibration bar: 50 µm).
Electron Microscopic Examination of Molars
By SEM, different regions of the dentin, including the circumpulpal and peripheral dentin regions were evident (Fig. 1A), yet no differences were observed between the two groups. By TEM, collagen fibrils oriented in different directions formed a dentin supporting structure in GI. These appeared relatively thick and densely arranged, and surrounded the openings of the dentinal tubules in a concentric manner (Fig. 5A). In GII, a clear reduction in both the diameter and the density of the collagen fibrils was observed, and fibrils lacked a concentric arrangement surrounding the openings of the dentinal tubules (Fig. 5B). Moreover, in GI, the odontoblastic process exhibited a higher density and homogeneity when compared to GII (Fig. 5C, D). Few fibrils were detected in the periodontoblastic space in both groups (Fig. 5A-D).
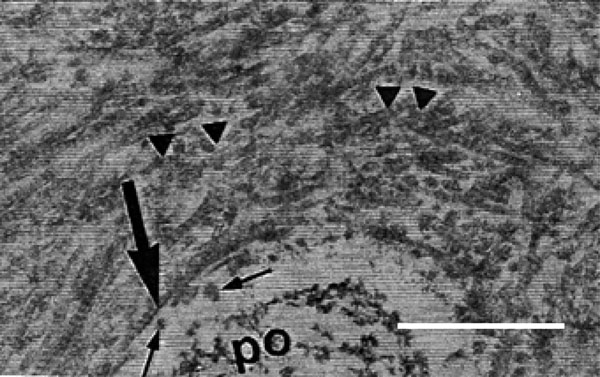
Transmission electron micrograph of rat molars. In GI molars, odontoblastic processes (op) were detected in the interior of the dentin tubules. Some residual fibrils (shorter arrows) were noted in the periodontoblastic space. Concentric collagen fibrils (larger arrow) were observed in the peritubular dentin and surrounding the dentin tubules (arrowheads). (Calibration bar: 1 µm).
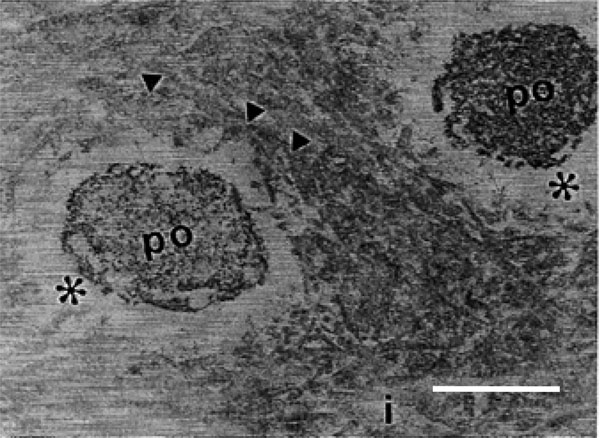
Transmission electron micrograph of rat molars. In GII molars, odontoblastic process (op), intertubular dentin (i), periodontoblastic space (*) were examined and collagen fibrils of the intertubular dentin exhibit differences (arrowheads) (Calibration bar: 1 µm).

Transmission electron micrograph of rat molars. Within GI molars, odontoblastic process and residual fibrils (arrows) exhibited dense and homogeneous structure (Calibration bar:0, 1 µm).

Transmission electron micrograph of rat molars. Within GII molars, the structure within the odontoblastic process appears less dense, and residual fibrils (arrows) can be detected (Calibration bar: 0,1 µm).
Measurement of Dentin Sagittal Sectional Surface Area
To quantify differences in dentin between the experimental groups, mean surface areas of molar sagittal sections were measured. Mean values (± standard deviation) were, 1.38 ± 0.29 mm2 for GI and 0.96 ± 0.19mm2 for GII, indicating 30% less dentin in molars from GII (Fig. 6). Statistical analysis revealed that this difference was significant (p = 0.018).
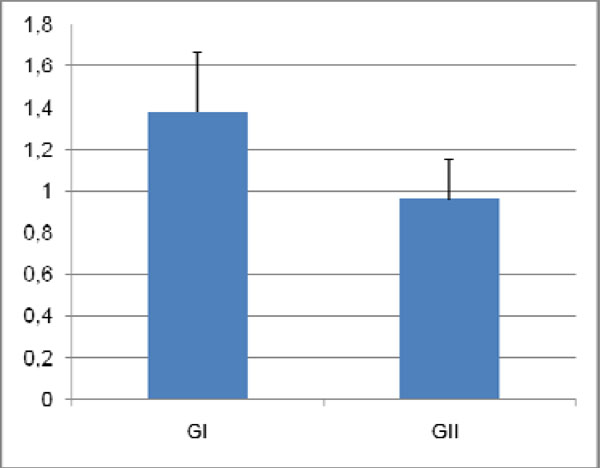
Surface area of dentin sections of molars from both groups. Mean values and standard deviations are represented.
DISCUSSION
To examine the effects of malnutrition in rat molar formation, we examined both external and ultra-structural morphological differences. Indeed, our results suggest that extrinsic factors, such as diet, can have a profound influence on tooth formation. Diet is thought to be important during two distinct stages of dental development [16]. During the pre-eruptive phase, nutrients are required for tooth maturation, contributing to their chemical composition, and influencing size and morphology. In addition, once teeth are formed, although they are no longer structurally altered, dental structure can be strongly influenced during its formation period. Our results are consistent with this proposal, since experimental malnutrition was induced during the pre-eruptive phase of tooth development. It is likely that malnutrition led to a delay in the development of the dentin in GII animals, which exhibited a predominance of type III collagen fibers. This can lead to consequences not only for tooth formation, but also mineralization of dental structures [28], since the collagen framework is thought not to be a passive participant in mineralization, but may play a more active role [10, 12, 14, 29, 30]. Previous studies of development and eruption of teeth in a variety of species have led to a consensus regarding the role of nutrition in timing of the eruption process. Although our experiments were performed during weaning period, morphological differences in the teeth of GII is consistent with studies correlating malnutrition and dental abnormalities [11], as well as development of caries [13,15].
Malnutrition is also associated to deficiencies in the metabolism of the connective tissue [31]. As a result, a significant decrease in collagen synthesis, the main protein constituent of the tissues, is observed, leading in turn to changes in growth in various organs. Importantly, this has also been found to influence the dental germs of rats submitted to pre and postnatal protein deficiency [20]. Thus, a reduction in collagen synthesis may explain the decrease in dentin observed in GII animals. This is consistent with previously published results examining molars from rat pups whose mothers were submitted to a protein deficient diet during gestation and lactation [10]. Importantly, reduced tooth size in undernourished animals is thought to result from a decrease in the volume of the dentin rather than a reduction in the thickness of the enamel [10, 22].
Protein deficiency led to smaller incisors than in the control animals [32]. Those authors suggested that the effect protein deficiency on dentin formation seems to be related to the predentin mineralization rates. Thus the odontoblasts, predentin and enamel appeared slower, but their ultrastructure were normal. Our results would appear to be at odds with some previously published reports. For example, protein deficiency later in development, led to no obvious differences in odontoblast structure [32]. In spite of this evidence, we observed irregular fibroblast morphology as well as its small and intensely stained nuclei in the teeth from the undernourished group, with malnutrition. These different results would be related to protein deficiency degree, because the authors [32] used protein deficiency later in development, during the 43rd day of life, and we verified this deficiency early, since the period of intra-uterine life until weaning. Moreover, cells within molars of the malnourished group appeared disorganized and non-palisade, in contrast with molars from the properly-nourished group. Disruption of the odontoblastic layer in GII may be responsible for the apparent decrease in the diameter and density of the collagen fibrils of the intertubular dentin.
Processes that affect collagen synthesis, such as protein or vitamin C deficiencies, can lead to periodontal ligament atrophy [33]. The irregularly arrayed collagen fibers of the periodontal ligament resulting from malnutrition, as well loss of epithelial adherence of the junctional epithelium are consistent with this proposal. The fibrilar part of this ligament has been reported to consist mainly of Type I collagen fibers [34], consistent with our findings. The clear prevalence of Type III collagen fibers in the malnourished group is therefore suggestive of immature periodontal ligaments, or delayed development in malnourished animals. The predominance of type III collagen in the alveolar bone in malnourished may also indicate a delay in tissue bone formation. This could result from a decrease in the osteoblastic activity, leading to a delay in the establishment of the ossification points. In fact, this phenomenon has been described in mandibles of rats submitted to protein and caloric deficiencies [7], and in humans [12, 15, 30] that lives in underdevelopment countries. In some places of Africa, this situation tends to be much more serious, and the prevalent oral diseases include cancrum oris, acute necrotising gingivitis, oral cancer, oral manifestations of HIV/AIDS, facial trauma, and lastly dental caries [35].
CONCLUSIONS
Protein deficiency led to reduction of Type I collagen and an increase in Type III collagen fibers in the dentin, periodontal ligaments, and alveolar bone of rats. In addition, protein deficiency led to greatly reduced dentin formation. This study suggests that both structural and ultra-structural features of the dentin-pulp complex and periodontal components of rat molars are affected by protein levels.


