All published articles of this journal are available on ScienceDirect.
Whole Saliva has a Dual Role on the Adherence of Candida albicans to Polymethylmetacrylate
Abstract
Adhesion of Candida albicans to acrylic of dental prostheses or to salivary macromolecules adsorbed on their surface is believed to be a critical event in the development of denture stomatitis. In previous studies our group has shown that adhesion of C. albicans germ tubes to polystyrene is decreased by saliva whereas C. albicans yeast cells adhesion to the same material is enhanced. The results presented in this study confirm this dual role played by whole saliva, since it decreased the adhesion of germ tubes but increased the adhesion of yeast cells to polymethylmetacrylate (PMMA). These effects mediated by whole saliva do not seem to be related to an inhibition of the germination of C. albicans, since similar levels of filamentation were observed in presence and absence of saliva. These results may give new insights into the conflicting role of saliva in the adhesion of C. albicans to acrylic resins of dental prostheses.
INTRODUCTION
Candida albicans is a dimorphic fungus that is commensal in the gastrointestinal and reproductive tracts of healthy individuals. Under certain predisposing conditions, C. albicans can convert into a pathogen capable of causing a variety of oral infections including pseudomembranous candidiasis, erythematous candidiasis and hyperplasic candidiasis, as well as Candida-associated denture stomatitis, Candida-associated angular cheilitis, rhomboid glossitis and chronic mucocutaneous candidiasis [1].
Candida-associated denture stomatitis is an inflammatory process that affects the oral mucosa of 25-65% of patients wearing removable dental prostheses [2]. The etiology is multifactorial consisting of either ill-fitting prostheses leading to mechanical irritation or poor hygiene leading to chronic infection. Regardless of the initiating process C. albicans is the major cause of fungal origin in denture stomatitis [3]. The first step implicated in denture stomatitis is adherence to acrylic or to salivary pellicles adsorbed on the surface of dental prosthesis being the most important event in the ability of C. albicans to colonize dentures in the mouth [4].
C. albicans can produce biofilms on natural surfaces, such as teeth, and foreign surfaces, such as prostheses. These biofilms are normally resistant to common antimicrobial therapy an increasing problem in clinics [5].
Saliva is the biologic fluid that bathes all oral surfaces and acts as a defense against microorganisms present in the oral cavity [6]. However, contradictory results have been obtained when the effect of saliva on the adherence of C. albicans to acrylic and plastic has been assessed. Several studies have shown that saliva reduces the adherence of C. albicans to plastic [7-9], a restorative material [10,11], PMMA [12,13] and epithelial cells [14] but others have observed that saliva enhances the adherence of C. albicans to PMMA [15], polystyrene [4] and epithelial cells [16]. In a previous paper, we have shown that whole saliva plays a different role in the adhesion of C. albicans to polystyrene depending on the morphological phase of C. albicans, since it enhanced the adhesion to polystyrene of yeast cells but decreased the adhesion of germinated cells [9]. In the present study, we have assessed the influence of whole saliva on adherence of C. albicans to PMMA in an attempt to extend our previous observation to the acrylic used to make dental prostheses.
MATERIALS AND METHODOLOGY
C. albicans serotype A (NCPF 3153), a filamentous strain obtained from the National Collection of Pathogenic Fungi (NCPF, Bristol, United Kingdom), C. albicans Ca2, a germ tube-deficient mutant of the parental strain serotype A NCPF 3153, kindly supplied by Dr. A. Cassone (Istituto Superiore di Sanita, Rome, Italy) and C. albicans UC1, an oral isolate from the Universidad de Cordoba (Argentina), were used in these experiments. The strains were maintained at 4 ºC on slants containing 20 g of glucose, 10 g of yeast extract, and 20 g of agar per liter.
The experimental protocols to obtain human saliva were approved by the Institutional Review Board of the School of Medicine and Odontology at the University of the Basque Country, Leioa, Spain, and the subjects gave their informed consent. Unstimulated whole saliva samples were collected and pooled from 5 healthy donors to eliminate sample variations. The donors had not taken any medication during the 3 months preceding the study and had no active periodontal disease or active caries. Saliva was centrifuged at 6000 g for 30 min at 4 ºC and the supernatant was stored at 4 ºC to be used the same day or stored at –80 ºC until used.
The acrylic strips for the adhesion assay were prepared as described by Samaranayake and MacFarlane [17], with some modifications. Briefly, transparent self-polymerizing acrylic powder (1.5 g of PMMA powder) was spread on an aluminium-foil-covered glass slide (2.5 x 7.5 cm). Monomer liquid (1ml) was poured on to the surface of the slide and immediately a second slide similar to the first was placed on top of the polymerizing mixture, and the slides were firmly secured at both ends with two binder clips. After bench curing for 30 min, the glass slides were separated. The resultant acrylic strips were cut into 5 x 5 mm squares and immersed in distilled water for 1 week to leach excess monomer. The strips were then ultrasonicated for 20 min, washed again in sterile distilled water, dried and used for the adhesion assay.
A modification of the method described by Tronchin et al. [18] was used in all adhesion experiments. Briefly, yeast cells were inoculated in medium 199, pH 6.7, at a final concentration of 8 x 105 cells/mL and were incubated for 2 h at 37 ºC or 25 ºC in 24-well tissue culture polystyrene plates containing the PMMA pieces and 350 µL of the yeast cell suspension. After incubation, the pieces were removed from the wells, washed with saline solution, and germination was quantified by counting the total number of cells and the number of germinated cells in 12 fields (0.64 mm2 each) per PMMA square by means of a graticule mounted in the focus of the ocular. The percentage of germination was calculated by the following equation: % Germination = (germinated cells/ total cells) x 100. PMMA pieces were washed three times with saline, and the adhesion was quantified by counting the total number of cells in the same 12 fields. The percentage of adhesion was calculated by the following equation: % adhesion = (adhered cells/ total cells) x 100. All values quoted represent mean figures derived from at least 4 independent assays. To determine the effect on adherence of the salivary pellicles, the PMMA pieces were previously incubated in 350 µL of whole saliva for 30 min at 37 ºC.
The ANOVA test was used to assess the significance of differences between means in adherence assays. Data were considered significant at P<0.05.
RESULTS
At 37 ºC and in the absence of human saliva, the adhesion of C. albicans NCPF 3153, UC1 and Ca2 to PMMA increased with time, reaching a maximum at 120 minutes (Fig. 1). However, the levels of adhesion were not the same for the three strains since C. albicans Ca2 reached only a 25% of adhesion whereas C. albicans NCPF 3153 and UC1 exceeded 90% of adhesion. At 25 ºC (Fig. 2), adherence of all three strains increased with time, although the percentages of adhesion reached were significantly lower than the ones observed at 37 ºC for strains C. albicans NCPF 3153 and UC1 (P<0.01 at 80 and 120 min) but similar to those observed for C. albicans Ca2.
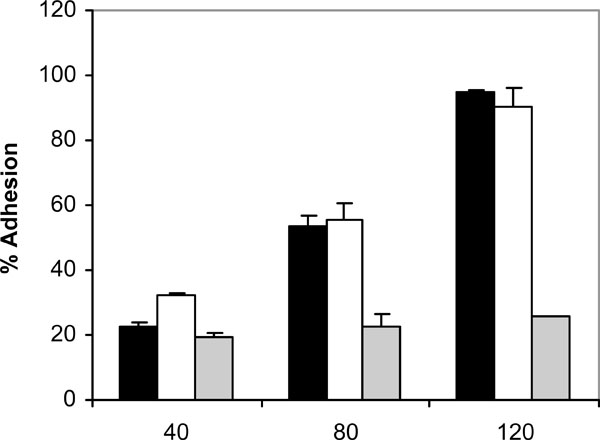
Adhesion of C. albicans NCPF 3153 (black), UC1 (white) and Ca2 (gray) to PMMA at 37ºC. Results represent the means of quadruplicate determinations ±SD.
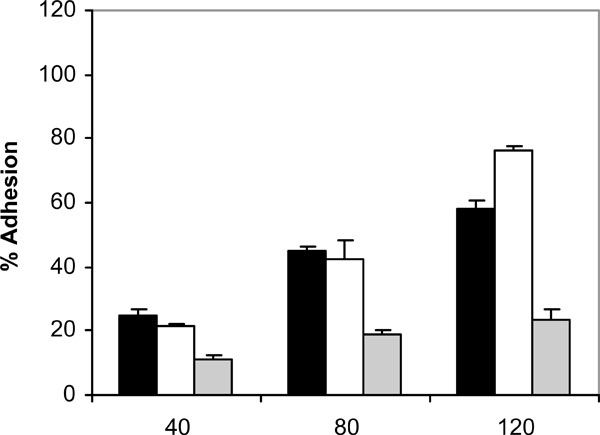
Adhesion of C. albicans NCPF 3153 (black), UC1 (white) and Ca2 (gray) to PMMA at 25ºC. Results represent the means of quadruplicate determinations ±SD.
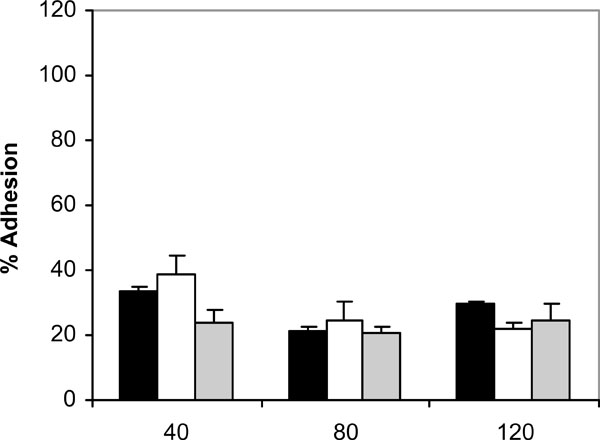
Adhesion of C. albicans NCPF 3153 (black), UC1 (white) and Ca2 (gray) to PMMA at 37ºC in presence of saliva. Results represent the means of quadruplicate determinations ±SD.
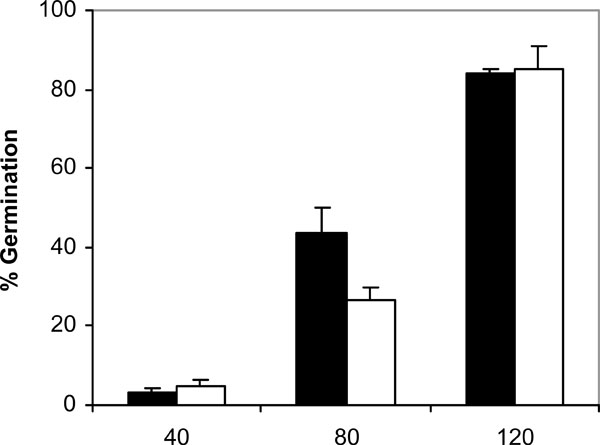
Germination of C. albicans NCPF 3153 (black) and UC1 (white) to PMMA at 37ºC. Results represent the means of quadruplicate determinations ±SD.
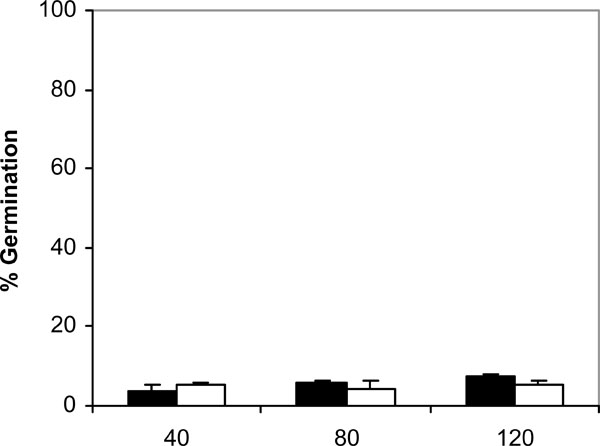
Germination of C. albicans NCPF 3153 (black) and UC1 (white) to PMMA at 25ºC. Results represent the means of quadruplicate determinations ±SD.
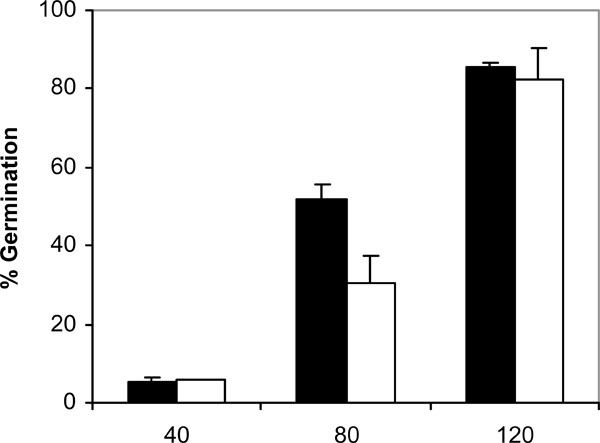
Germination of C. albicans NCPF 3153 (black) and UC1 (white) to PMMA at 37ºC in presence of saliva. Results represent the means of quadruplicate determinations ±SD.
Addition of saliva to the adhesion assay changed the kinetics of adhesion observed in absence of saliva (Fig. 3). At 40 min of incubation at 37ºC, the adherence observed for all the strains was higher than the adherence in the absence of saliva at the same period of time, with a rise in adhesion ranging from 11.4% (C. albicans NCPF 3153) to 4.2% (C. albicans Ca2). However, at 80 and 120 min in the presence of saliva the adhesion of C. albicans to PMMA was significantly reduced (P<0.001).
Since adhesion of C. albicans to plastic materials has been shown to be related with germination [18,19], we assessed the filamentation in the conditions of the adhesion assay. At 37ºC and in absence of saliva, germination increased with time in C. albicans NCPF 3153 and UC1 (Fig. 4). At 40 min of incubation the majority of the C. albicans cells presented yeast morphology and only 3% showed short germ tubes. Longer periods of incubation produced an increase in the percentage of germination and an extension in the length of the germ tubes. The maximum level of germination, which was similar for both strains reaching 85% at 120 min. However, germination at 25ºC (Fig. 5) was very low and remained fairly constant through the time of incubation studied (4-7%). As expected, C. albicans Ca2 did not germinate at 25 or 37ºC in presence or absence of saliva (data not shown). In the presence of saliva (Fig. 6), germination also increased with time showing levels similar to those reached by the controls in absence of saliva.
DISCUSSION
C. albicans adheres to a variety of surfaces in the oral cavity that are constantly bathed by saliva [6]. Saliva has a defensive role in the oral cavity that is non-specific (mucins, lysozyme, peroxydase, histatins, etc.) and specific (secretory IgA). Mucins enhance agglutination preventing colonization [20, 21] whereas lysozyme, peroxydase [6] and histatins [22, 23] are candidacidal. On the other hand, saliva provides water, nutrients and adherence factors [6]. A number of studies have shown that the adherence of C. albicans to acrylic materials [4,7-9,15] can be modulated by saliva. However, the precise role of saliva on the adhesion of C. albicans to acrylic is not clear, since both an increase and a decrease in adhesion have been described [4,7,8]. A possible explanation for this contradictory effect has been proposed by San Millán et al. [9] when studying the adhesion of C. albicans to polystyrene, since whole saliva decreased or enhanced the adhesion of C. albicans to polystyrene depending on the morphological phase of C. albicans. Thus, it is possible that whole saliva plays a dual role on the adhesion of C. albicans to plastic materials used to make dental prostheses, decreasing adhesion of germinated cells and enhancing the adhesion of yeast cells.
The results presented in this study confirm this dual role played by whole saliva, since it decreased the adhesion of germ tubes to PMMA but increased the adhesion of yeast cells to the acrylic. Although germination is an important factor in the adhesion of C. albicans to plastic surfaces and epithelial cells [18, 24] and in this study we have observed that adhesion increases in parallel with germ tube formation, the effects mediated by whole saliva do not seem to be related to an inhibition of the germination of C. albicans, since similar levels of filamentation were observed in presence and absence of saliva. Secretory IgA and possibly other salivary components are likely to modulate the adhesion of C. albicans to PMMA by binding the cell wall surface of the fungus. In the inhibitory effect, binding of the cell wall will mask the fungal adhesins, while the enhancement of adhesion may be mediated by a reorganization of the cell wall adhesins induced by certain types of antibodies [10,11,19]. Interestingly, the dual effect caused by whole saliva may be specific of plastic materials since it was not observed with the composite Herculite, where only the inhibitory effect on the adhesion of C. albicanswas observed [11].
Future studies should be aimed at finding antimicrobial agents that can decrease C. albicans adhesion to PMMA or kill C. albicans adhered to PMMA surface. In this sense, more studies like the one performed recently by Manfredi et al. [25] showing that a synthetic killer peptide has potential candidacidal effect on C. albicans cells adhered to acrylic surfaces should be done.
CONCLUSIONS
In conclusion, the results presented in this study demonstrate that whole saliva decreases the adherence of C. albicans to PMMA, although our results show it plays a dual role that depends on the morphological phase of the fungus.


